Deposition Date
2020-04-14
Release Date
2020-12-30
Last Version Date
2024-01-24
Entry Detail
PDB ID:
6YO3
Keywords:
Title:
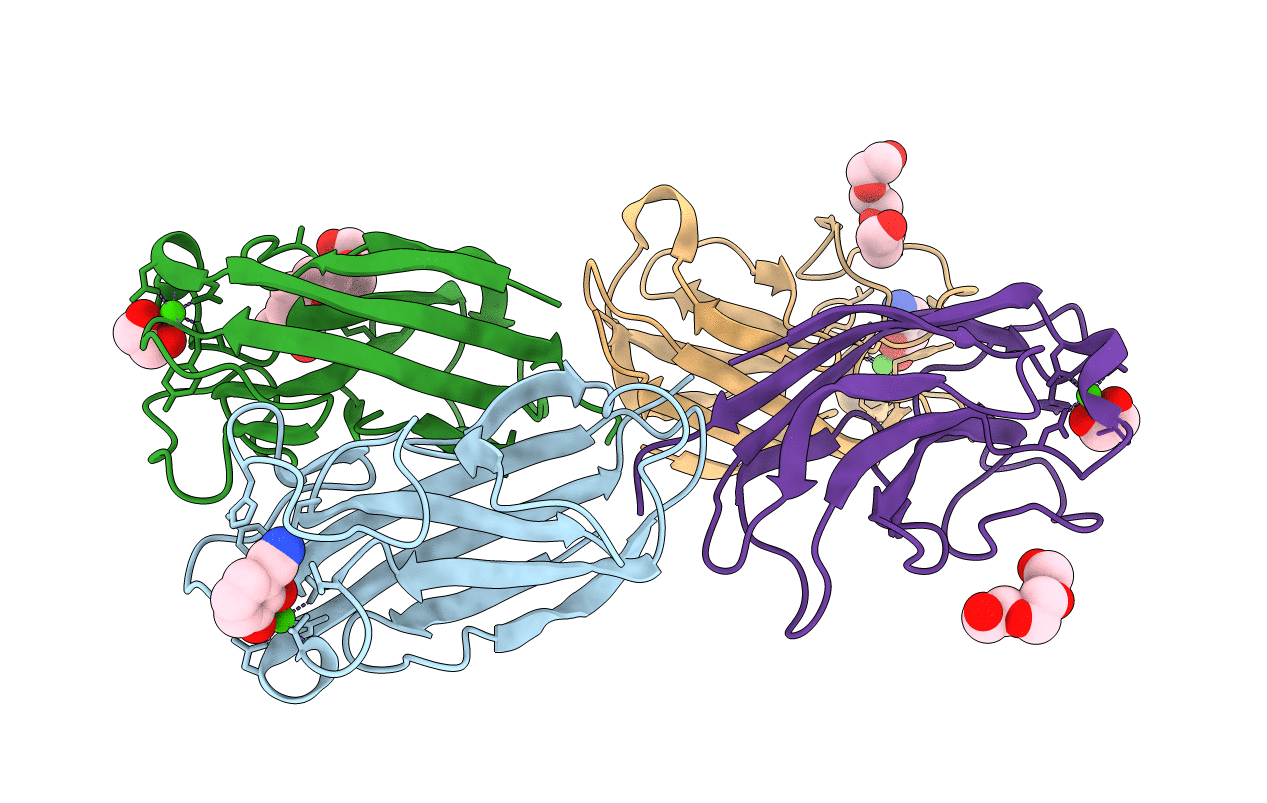
LecA from Pseudomonas aeruginosa in complex with a catechol CAS no. 67984-81-0
Biological Source:
Source Organism:
Host Organism:
Method Details:
Experimental Method:
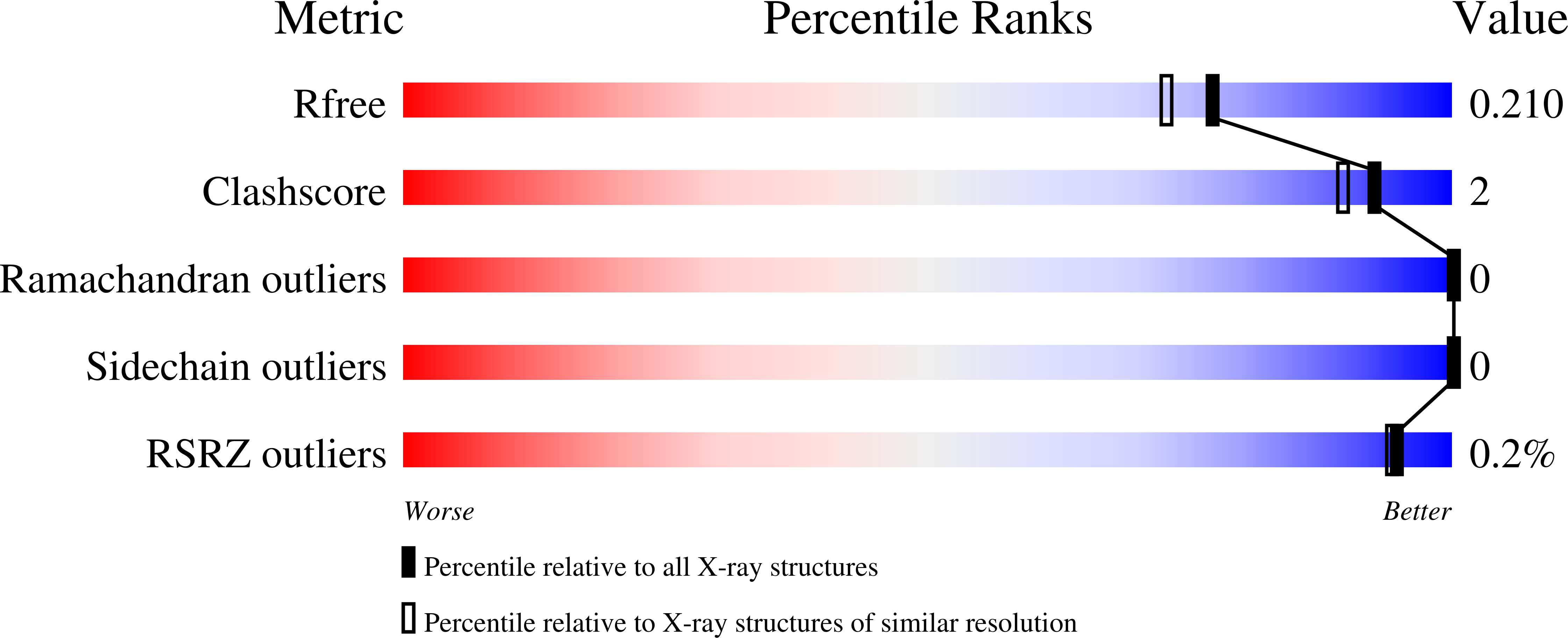
Resolution:
1.84 Å
R-Value Free:
0.20
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
I 1 2 1