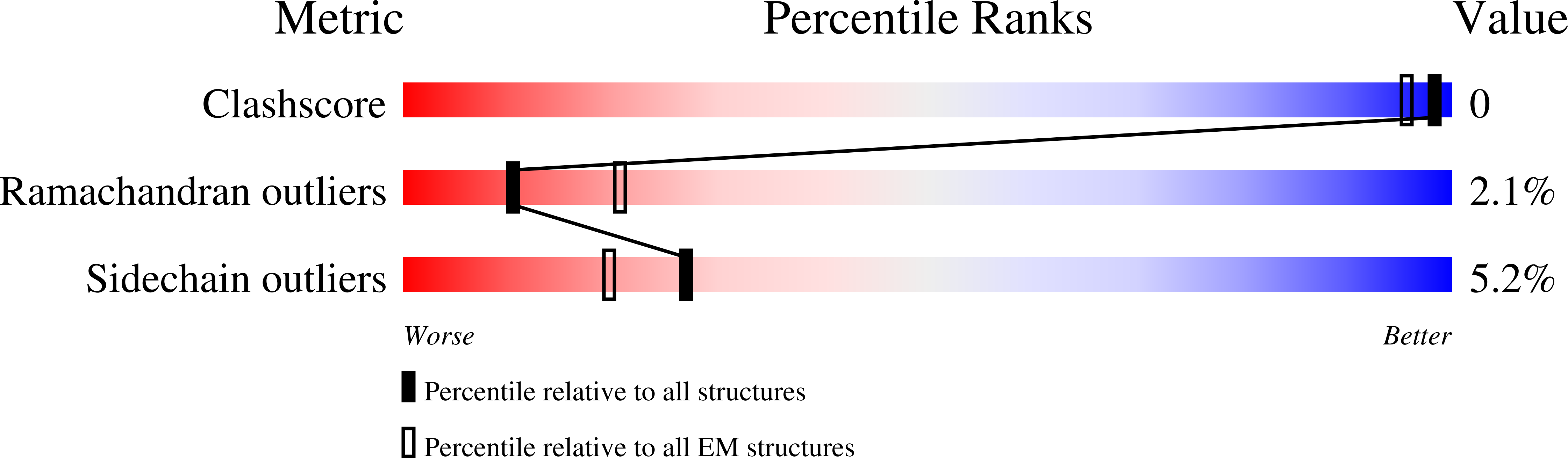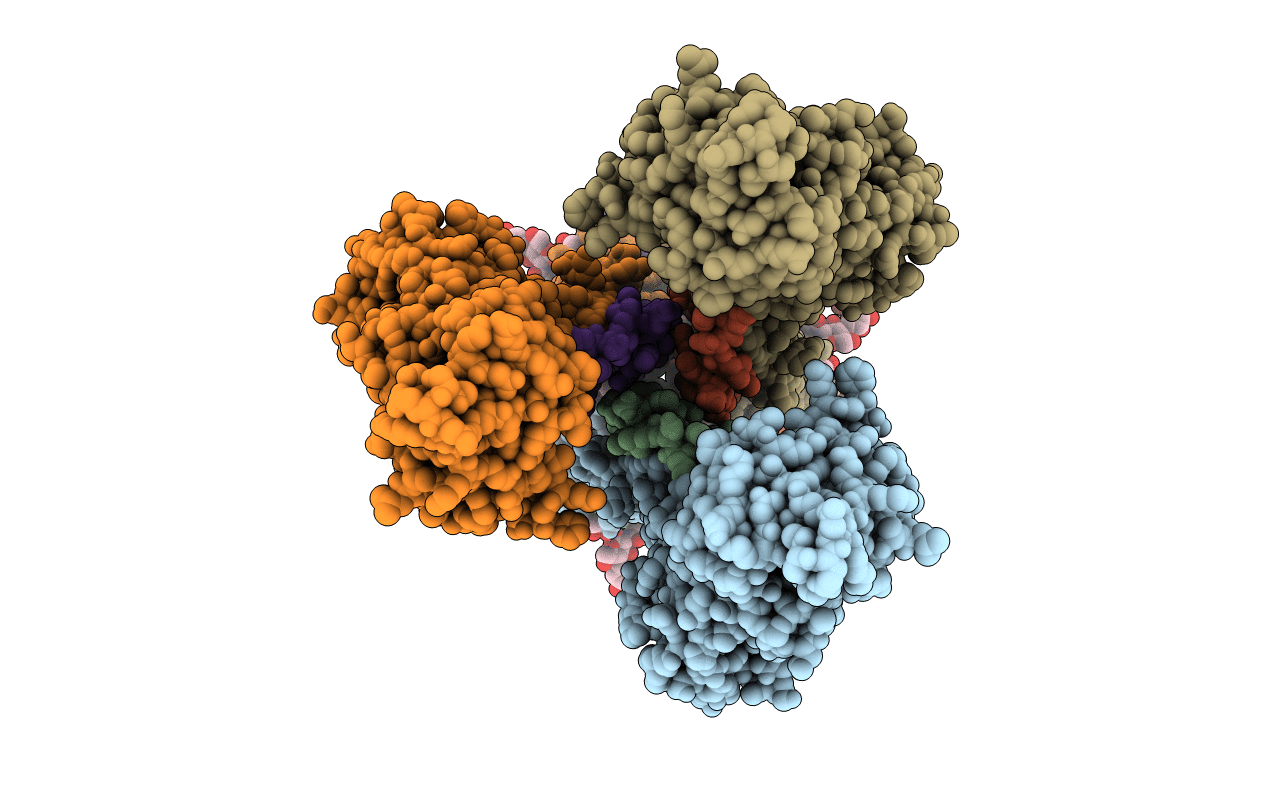
Deposition Date
2020-03-31
Release Date
2021-03-24
Last Version Date
2024-10-16
Entry Detail
PDB ID:
6YI5
Keywords:
Title:
In-situ structure of the trimeric HEF from influenza C by flexible fitting into a cryo-ET map.
Biological Source:
Source Organism(s):
Method Details:
Experimental Method:
Resolution:
9.10 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SUBTOMOGRAM AVERAGING