Deposition Date
2020-03-27
Release Date
2020-09-16
Last Version Date
2024-01-24
Entry Detail
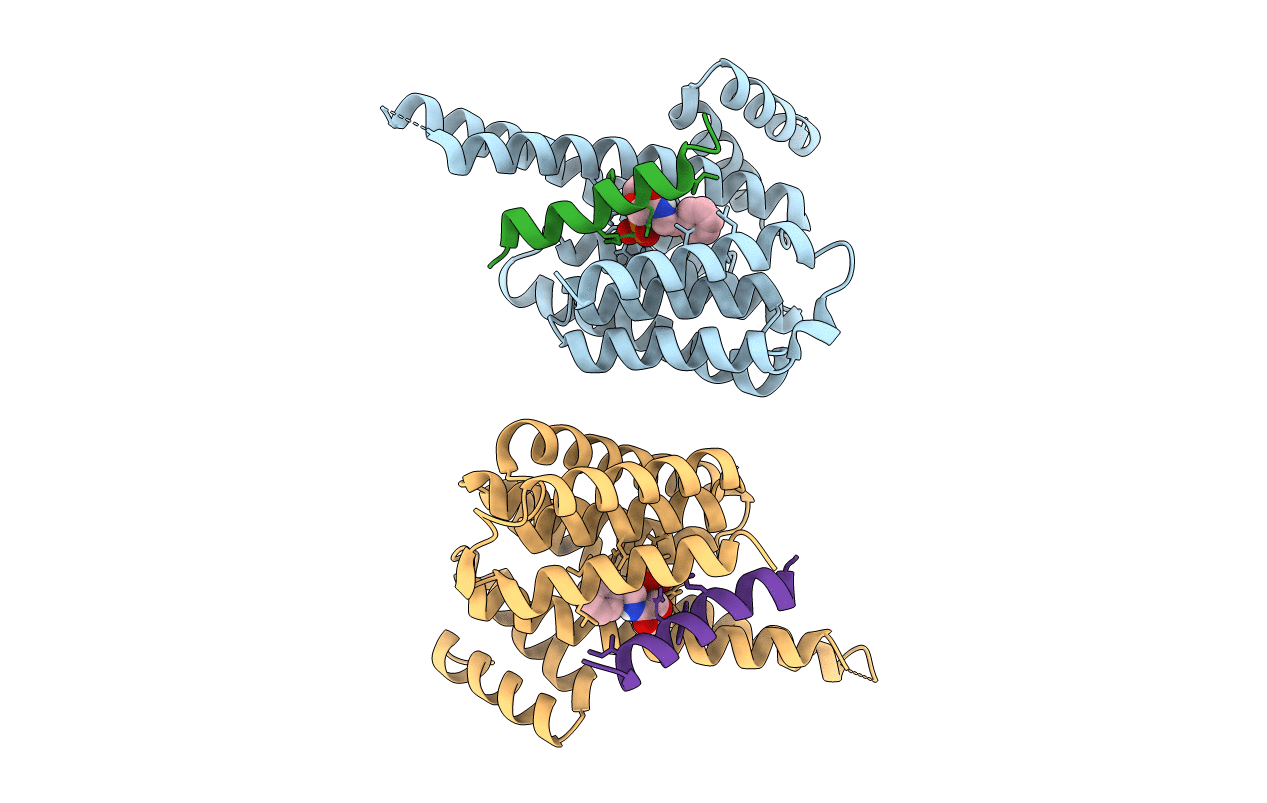
PDB ID:
6YGJ
Keywords:
Title:
small-molecule stabilizer of 14-3-3 and the Carbohydrate Response Element Binding Protein (ChREBP) protein-protein interaction
Biological Source:
Source Organism:
Homo sapiens (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
Resolution:
2.07 Å
R-Value Free:
0.27
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
C 1 2 1


