Deposition Date
2020-03-06
Release Date
2020-07-22
Last Version Date
2024-11-20
Entry Detail
PDB ID:
6Y98
Keywords:
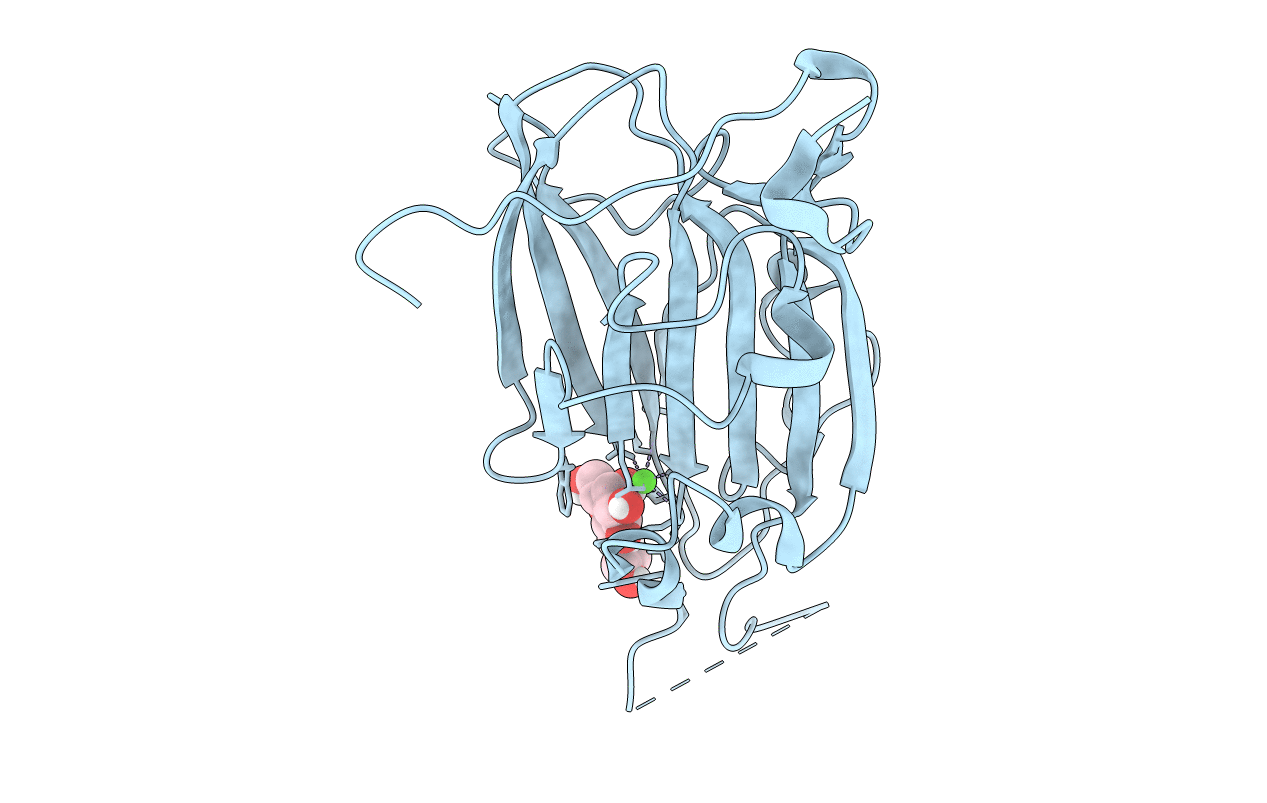
Title:
Crystal Structure of subtype-switched Epithelial Adhesin 9 to 1 A domain (Epa9-CBL2Epa1) from Candida glabrata in complex with beta-lactose
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
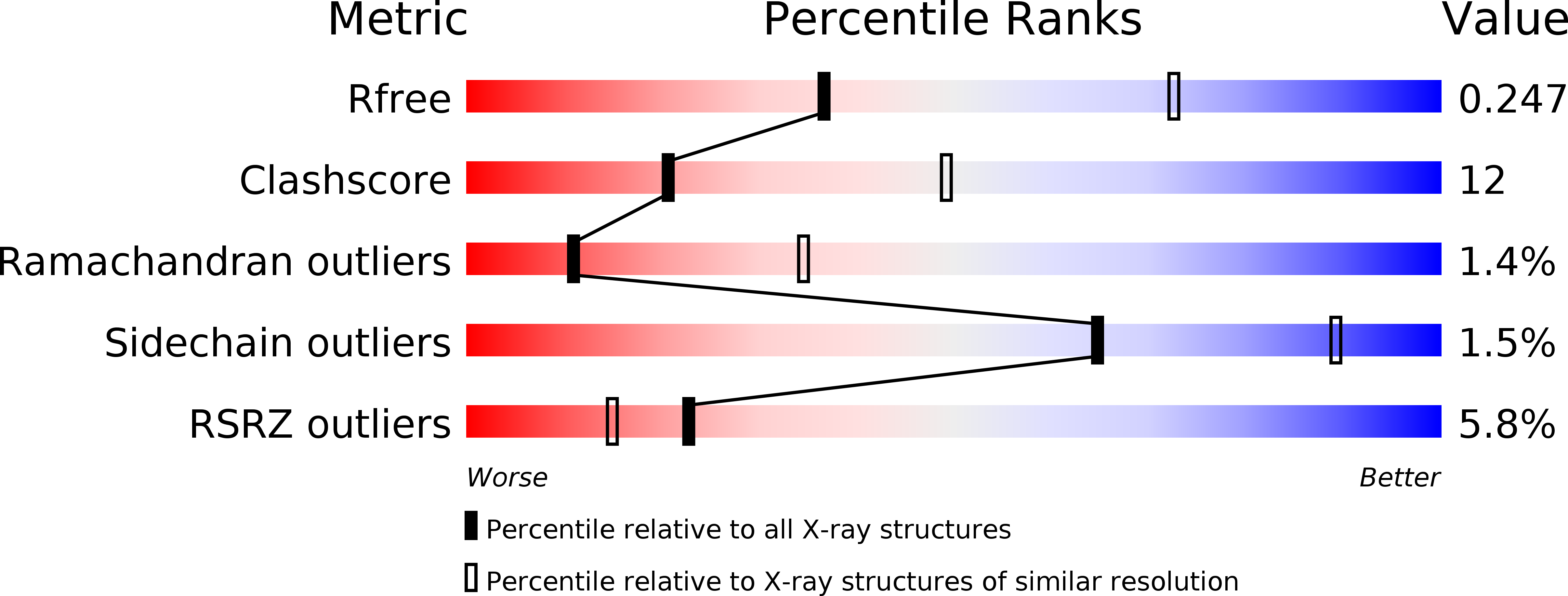
Resolution:
2.80 Å
R-Value Free:
0.24
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 31 2 1