Deposition Date
2020-07-13
Release Date
2020-12-30
Last Version Date
2024-10-23
Entry Detail
PDB ID:
6XRJ
Keywords:
Title:
Crystal structure of the disulfide linked DH717.1 Fab dimer, derived from a macaque HIV-1 vaccine-induced Env glycan-reactive neutralizing antibody B cell lineage
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
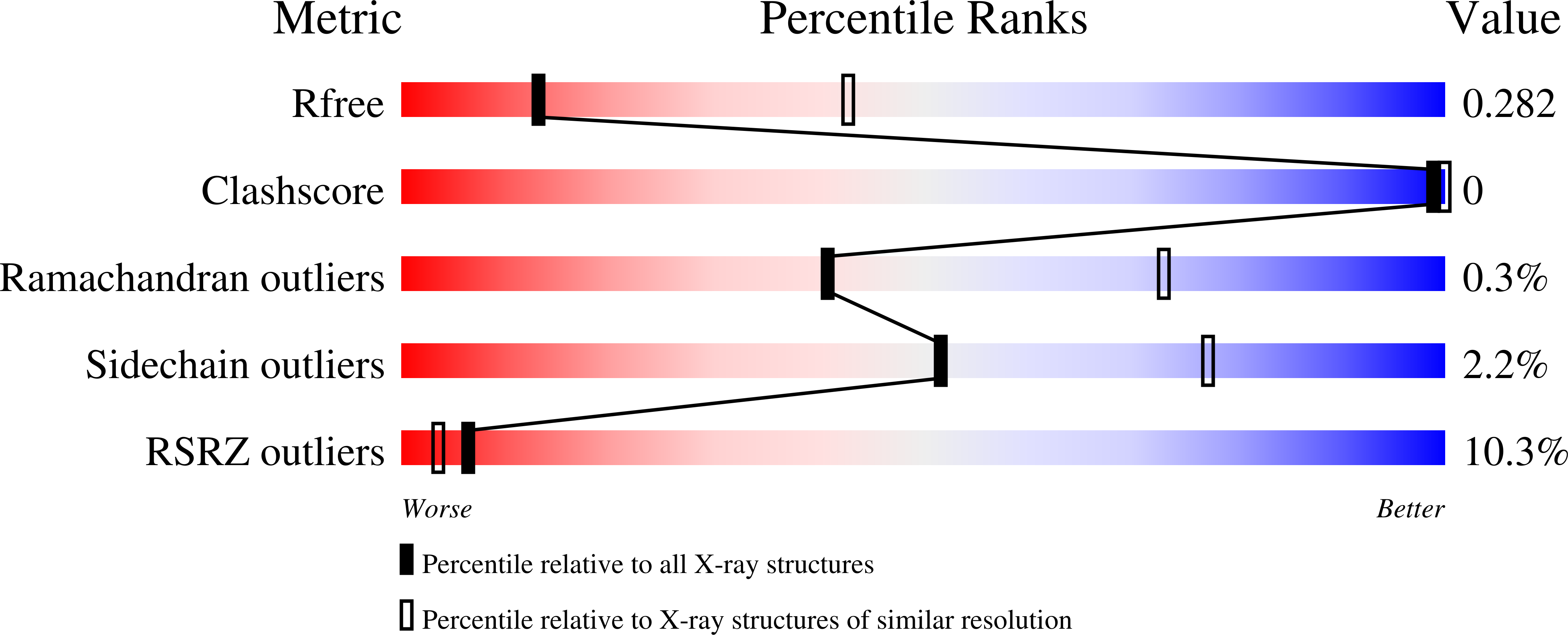
Resolution:
3.15 Å
R-Value Free:
0.28
R-Value Work:
0.24
R-Value Observed:
0.24
Space Group:
P 43 2 2