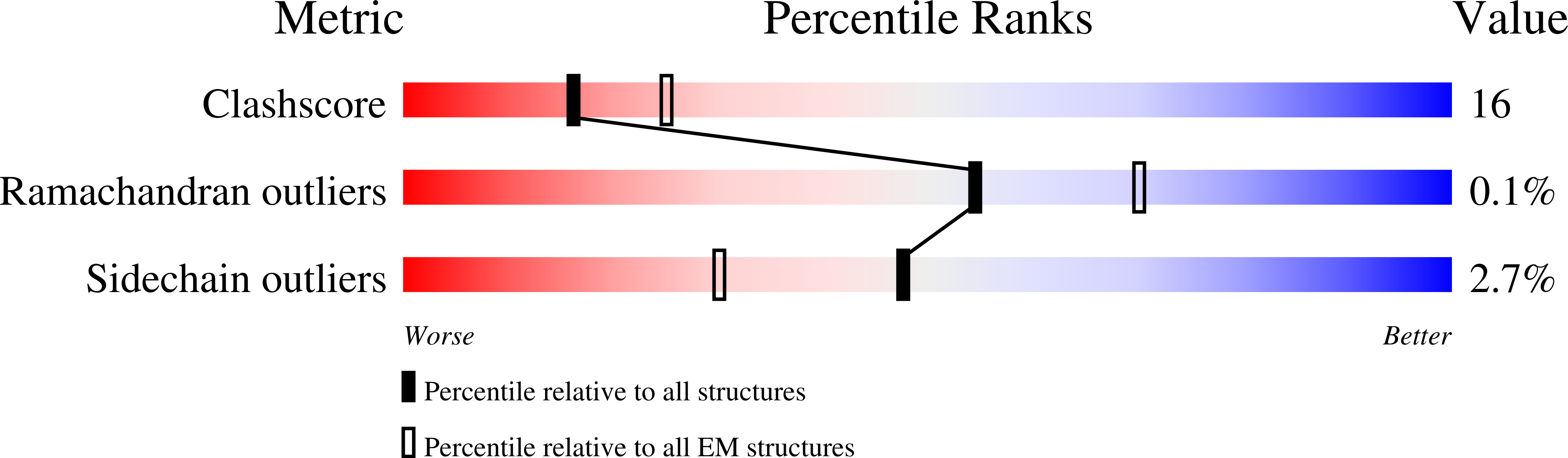Deposition Date
2020-07-05
Release Date
2020-08-26
Last Version Date
2024-03-06
Entry Detail
PDB ID:
6XNX
Keywords:
Title:
Structure of RAG1 (R848M/E649V)-RAG2-DNA Strand Transfer Complex (Dynamic-Form)
Biological Source:
Source Organism:
Mus musculus (Taxon ID: 10090)
Homo sapiens (Taxon ID: 9606)
Homo sapiens (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
Resolution:
2.70 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE