Deposition Date
2020-06-21
Release Date
2020-10-07
Last Version Date
2024-10-16
Entry Detail
PDB ID:
6XIS
Keywords:
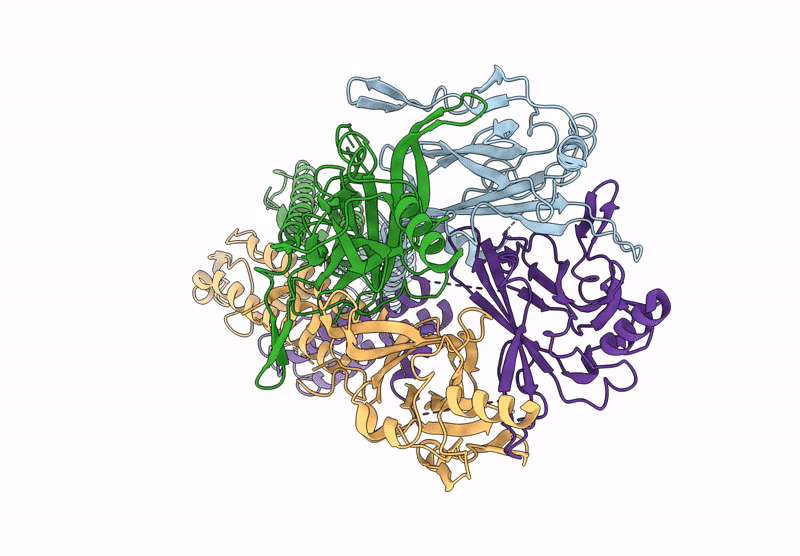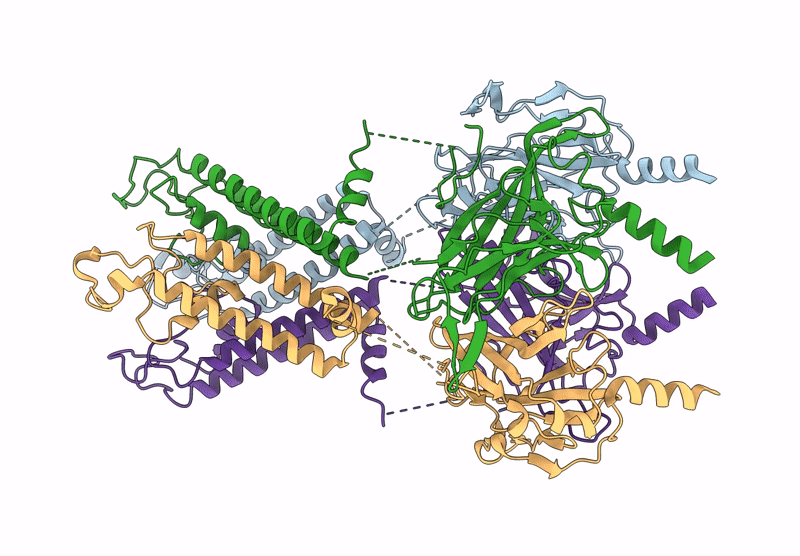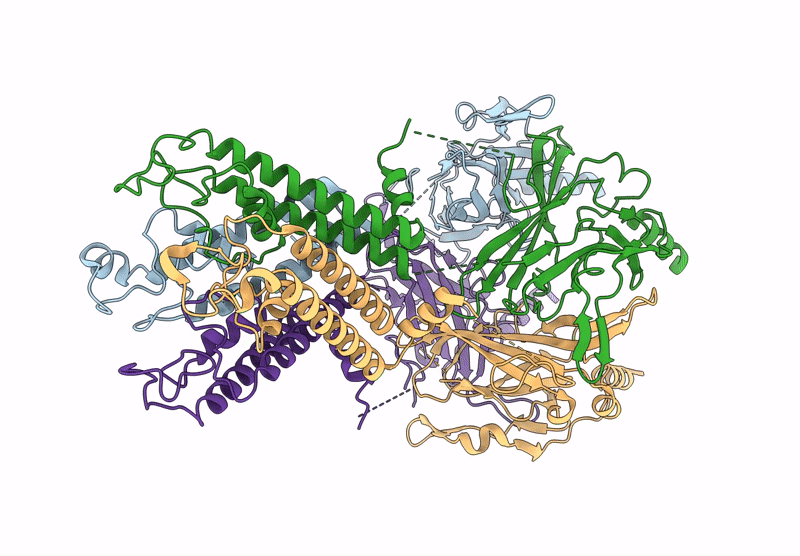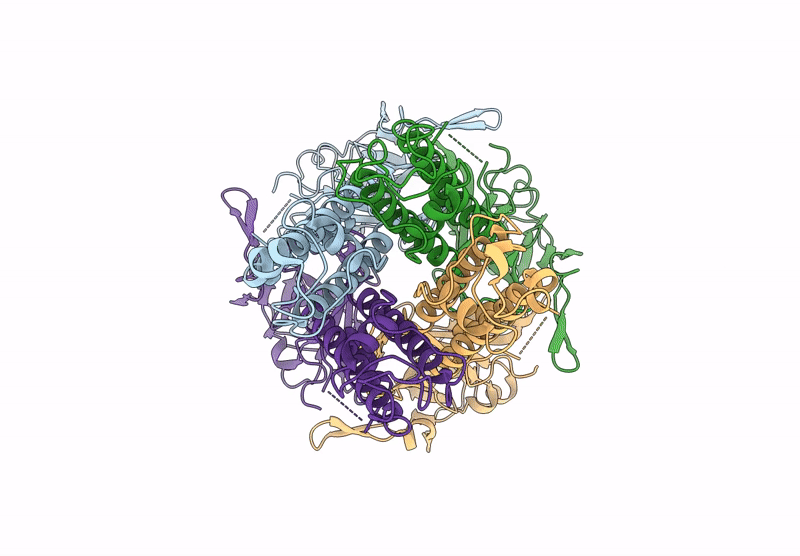
Title:
Cryo-EM structure of the G protein-gated inward rectifier K+ channel GIRK2 (Kir3.2) in apo form
Biological Source:
Source Organism(s):
Mus musculus (Taxon ID: 10090)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.90 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


