Deposition Date
2020-06-19
Release Date
2020-07-08
Last Version Date
2024-11-06
Entry Detail
PDB ID:
6XHL
Keywords:
Title:
Covalent complex of SARS-CoV main protease with N-[(2S)-1-({(2S,3S)-3,4-dihydroxy-1-[(3S)-2-oxopyrrolidin-3-yl]butan-2-yl}amino)-4-methyl-1-oxopentan-2-yl]-4-methoxy-1H-indole-2-carboxamide
Biological Source:
Source Organism:
Human SARS coronavirus (Taxon ID: 694009)
Host Organism:
Method Details:
Experimental Method:
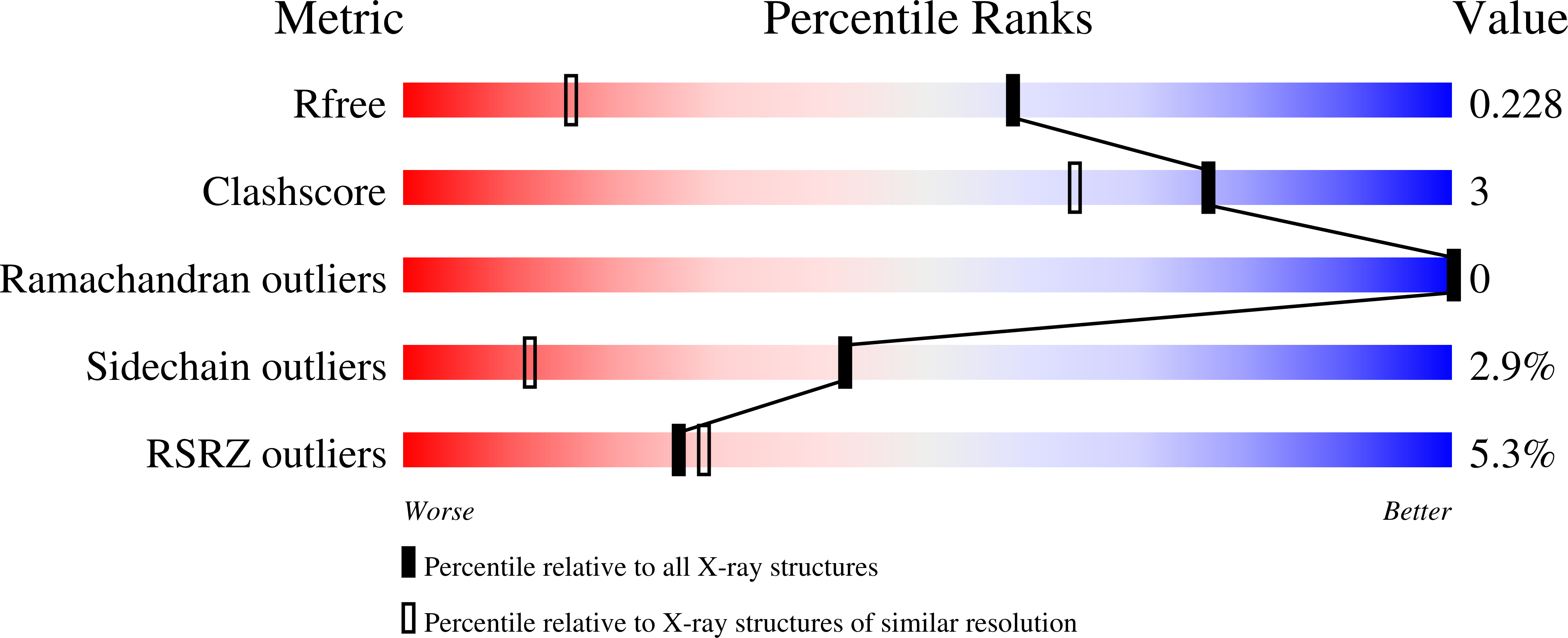
Resolution:
1.47 Å
R-Value Free:
0.22
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 1 21 1