Deposition Date
2020-06-05
Release Date
2020-06-17
Last Version Date
2024-10-23
Entry Detail
PDB ID:
6XB2
Keywords:
Title:
Room temperature X-ray crystallography reveals catalytic cysteine in the SARS-CoV-2 3CL Mpro is highly reactive: Insights for enzyme mechanism and drug design
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
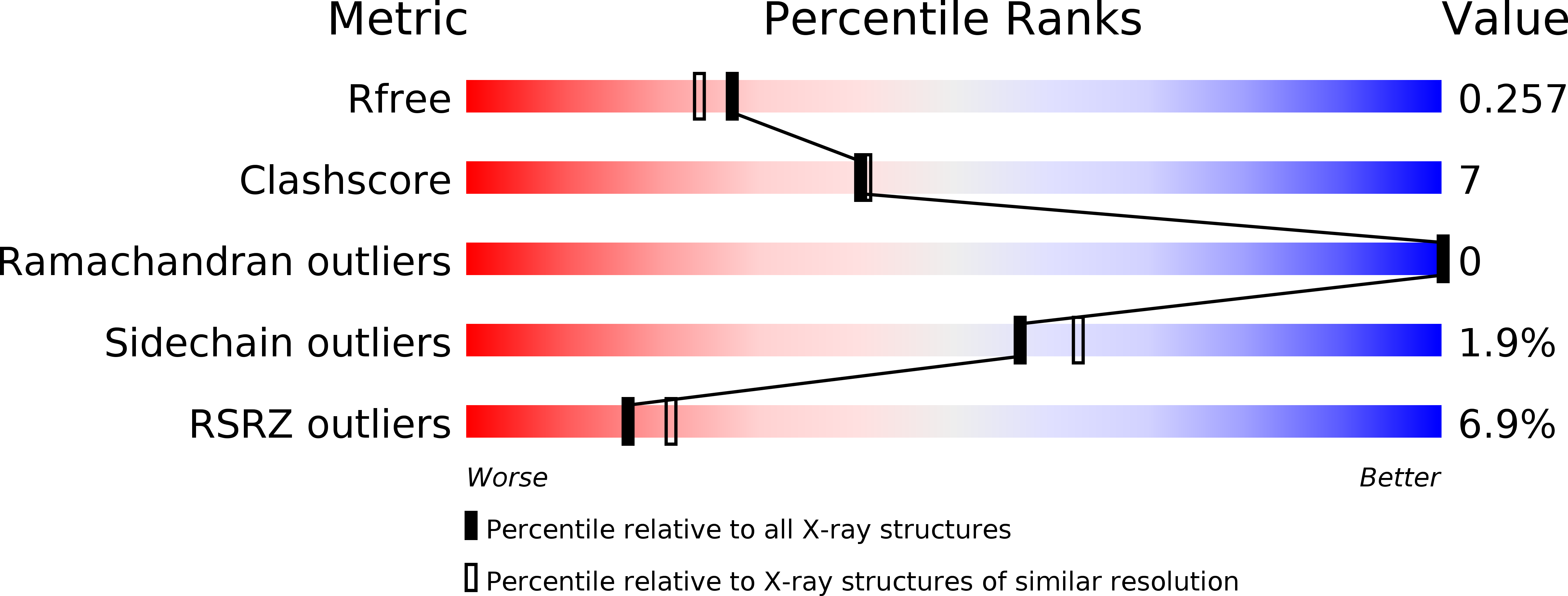
Resolution:
2.10 Å
R-Value Free:
0.25
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
I 1 2 1