Deposition Date
2020-05-18
Release Date
2021-03-03
Last Version Date
2023-10-18
Entry Detail
PDB ID:
6X1I
Keywords:
Title:
Two-Component D3 Assembly Constructed by Fusing Symmetric Oligomers to Coiled Coils
Biological Source:
Source Organism:
Host Organism:
Method Details:
Experimental Method:
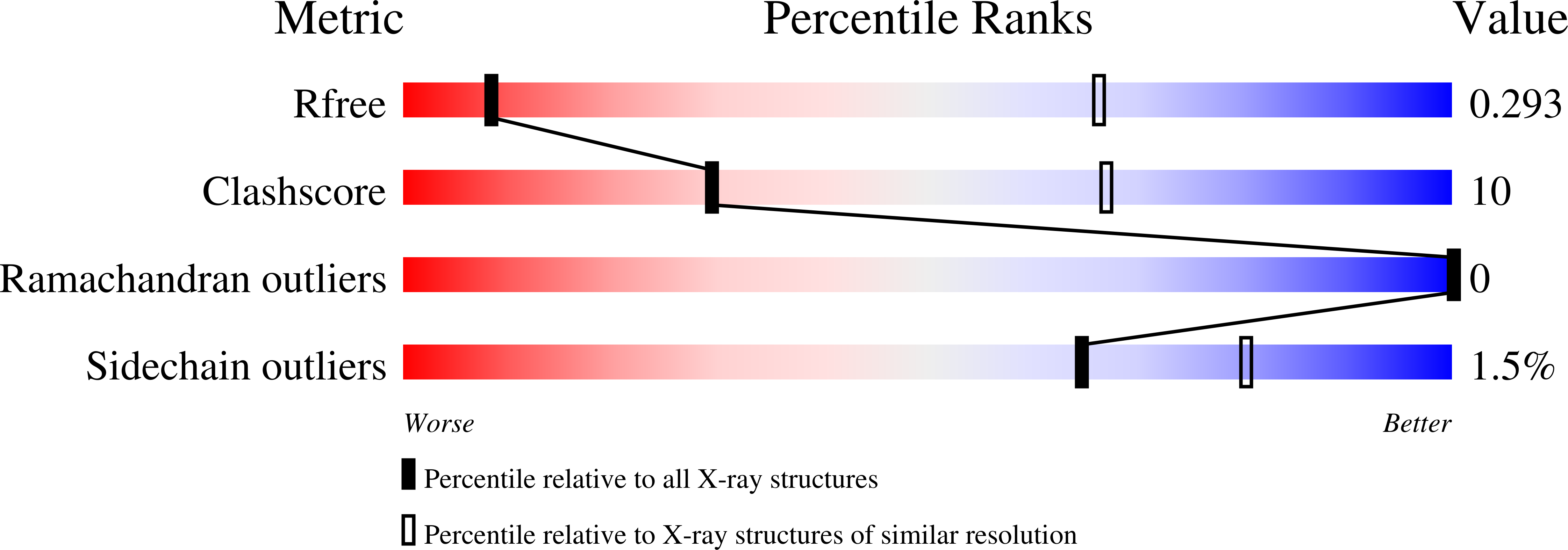
Resolution:
4.32 Å
R-Value Free:
0.29
R-Value Work:
0.27
R-Value Observed:
0.27
Space Group:
P 43 3 2