Deposition Date
2020-05-13
Release Date
2021-05-19
Last Version Date
2023-10-18
Entry Detail
PDB ID:
6WYN
Keywords:
Title:
Transition metal inhibition and structural refinement of the M. tuberculosis esterase, Rv0045c
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
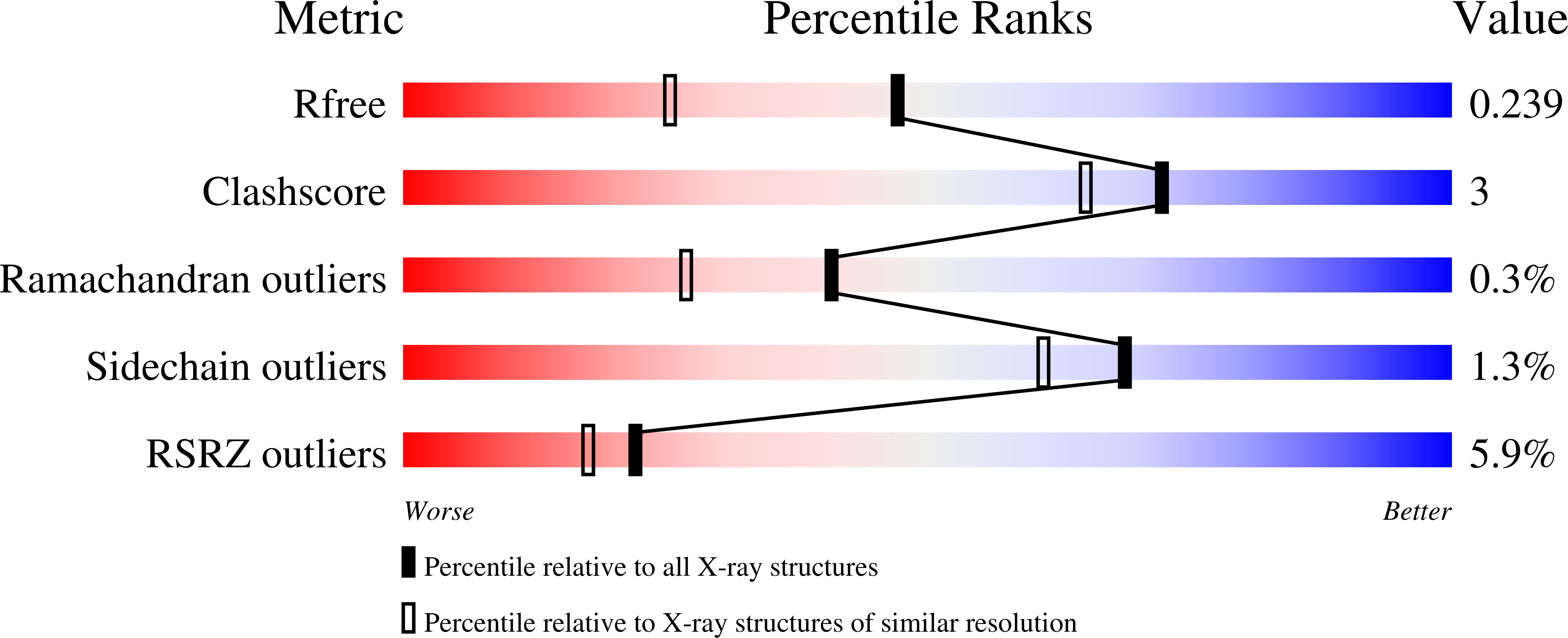
Resolution:
1.81 Å
R-Value Free:
0.23
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 31 2 1