Deposition Date
2020-04-29
Release Date
2020-06-03
Last Version Date
2025-06-04
Entry Detail
PDB ID:
6WQN
Keywords:
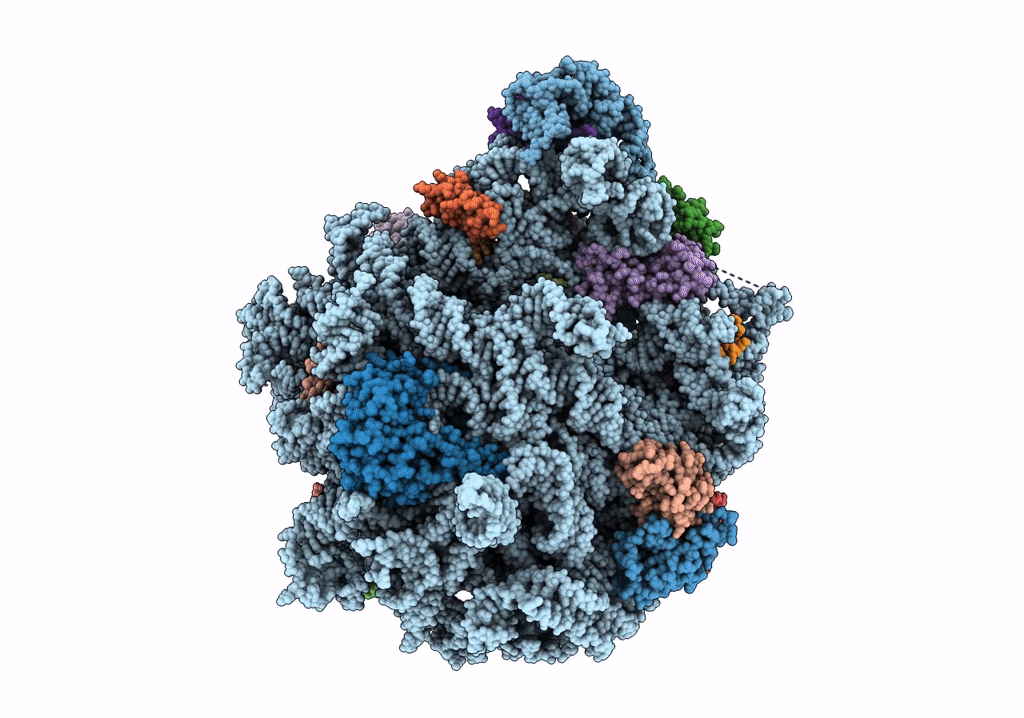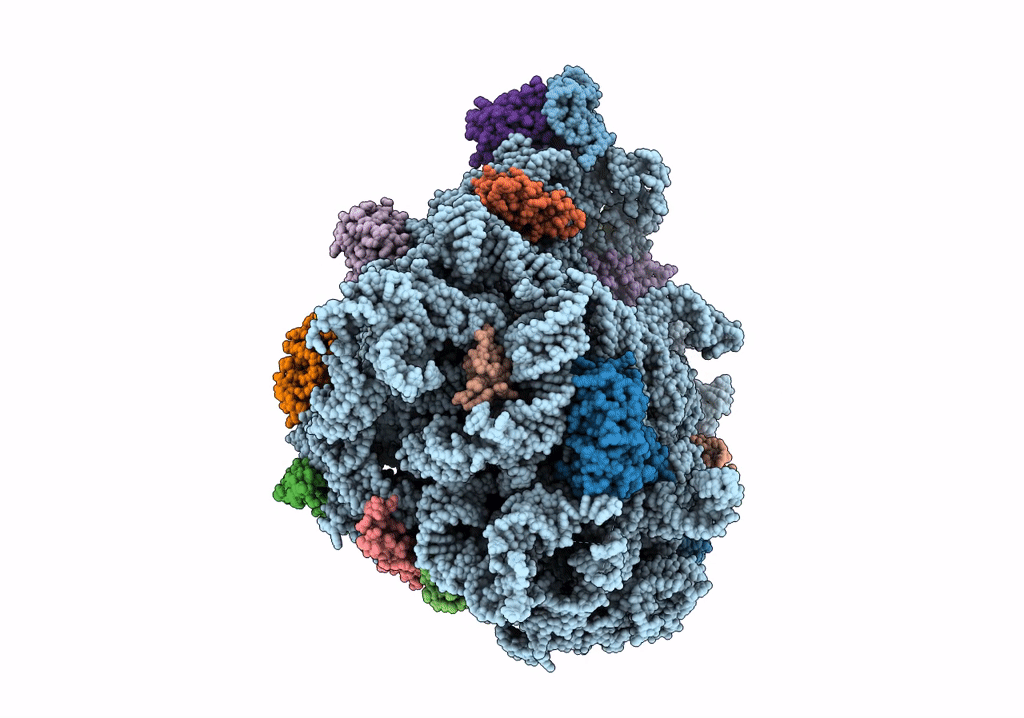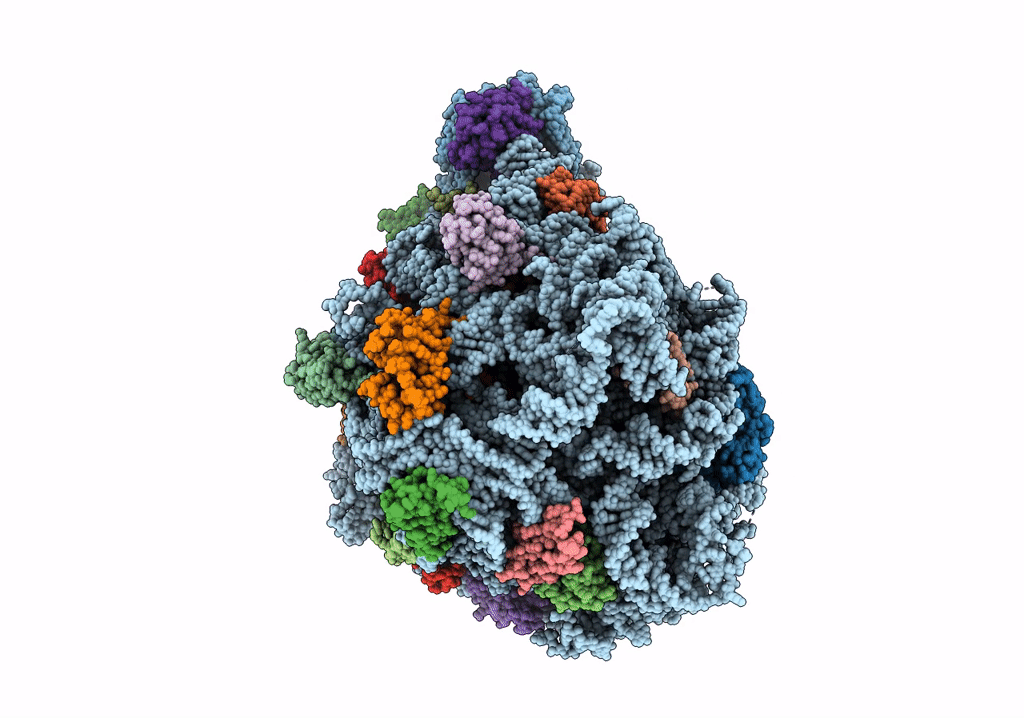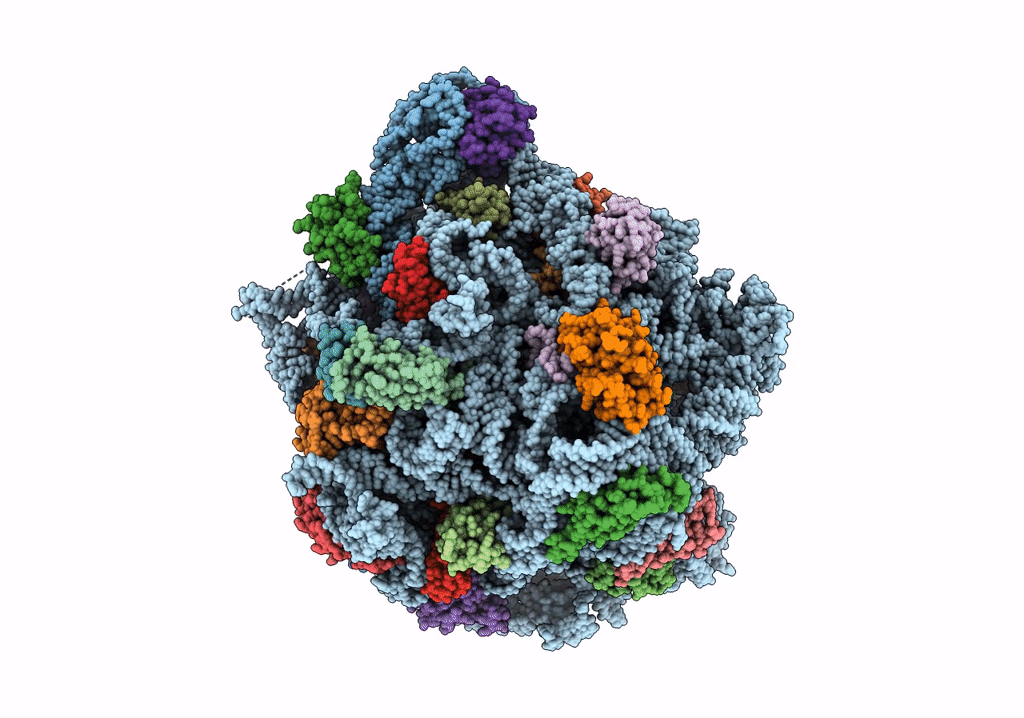
Title:
Structure of the 50S subunit of the ribosome from Methicillin Resistant Staphylococcus aureus in complex with the antibiotic, contezolid
Biological Source:
Source Organism(s):
Staphylococcus aureus (Taxon ID: 1280)
Method Details:
Experimental Method:
Resolution:
2.90 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


