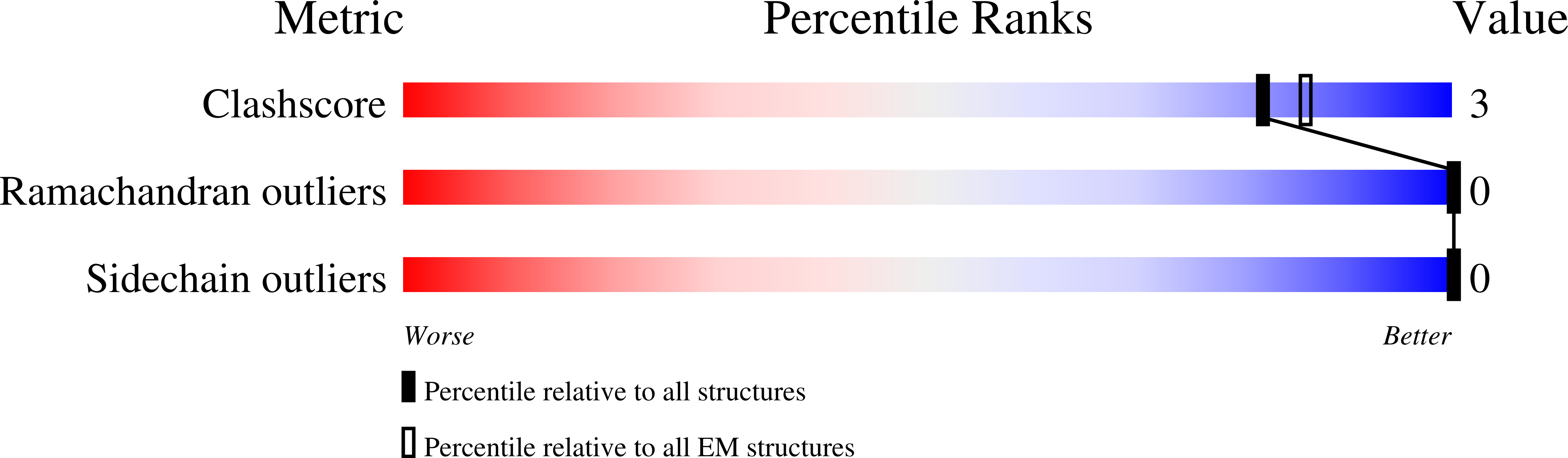
Deposition Date
2020-04-17
Release Date
2020-12-02
Last Version Date
2025-05-28
Method Details:
Experimental Method:
Resolution:
4.20 Å
Aggregation State:
FILAMENT
Reconstruction Method:
HELICAL