Deposition Date
2020-04-13
Release Date
2020-04-22
Last Version Date
2023-10-25
Entry Detail
PDB ID:
6WJI
Keywords:
Title:
2.05 Angstrom Resolution Crystal Structure of C-terminal Dimerization Domain of Nucleocapsid Phosphoprotein from SARS-CoV-2
Biological Source:
Source Organism:
Host Organism:
Method Details:
Experimental Method:
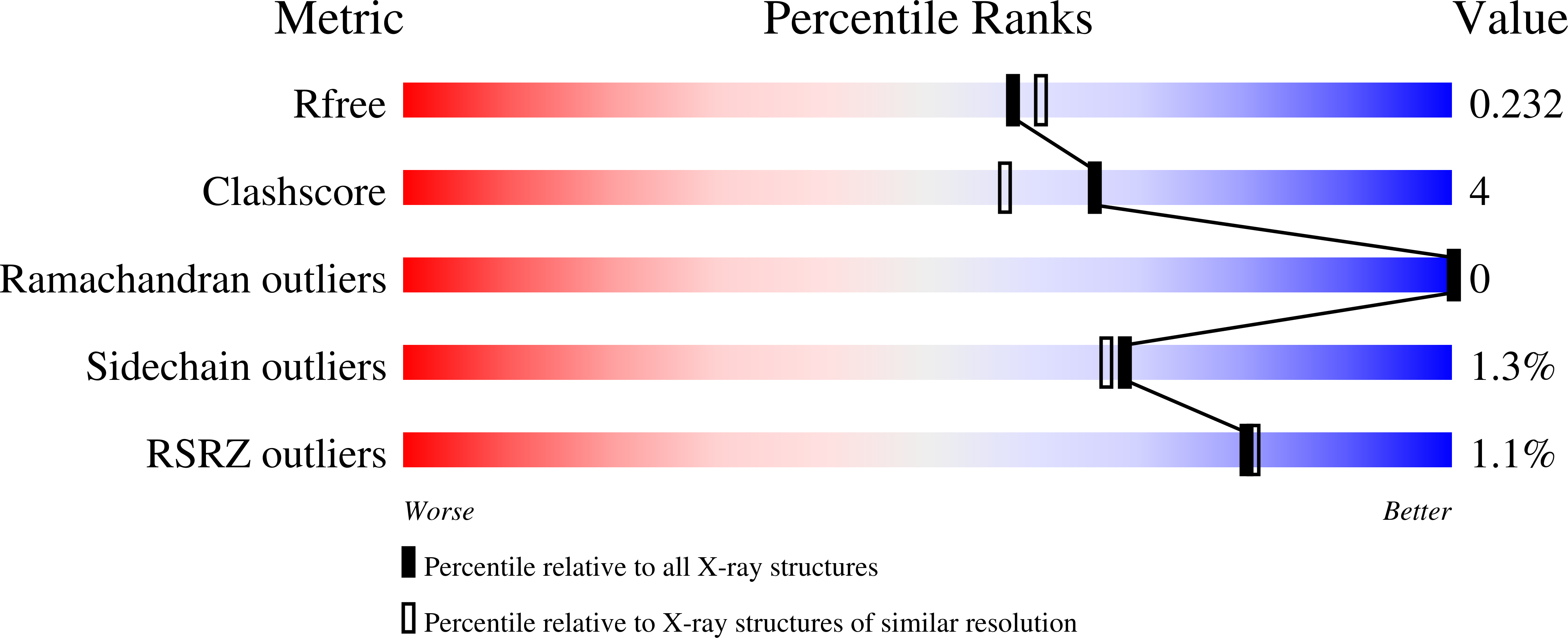
Resolution:
2.05 Å
R-Value Free:
0.22
R-Value Work:
0.18
Space Group:
P 21 21 21