Deposition Date
2020-03-22
Release Date
2020-05-13
Last Version Date
2025-04-02
Entry Detail
PDB ID:
6W98
Keywords:
Title:
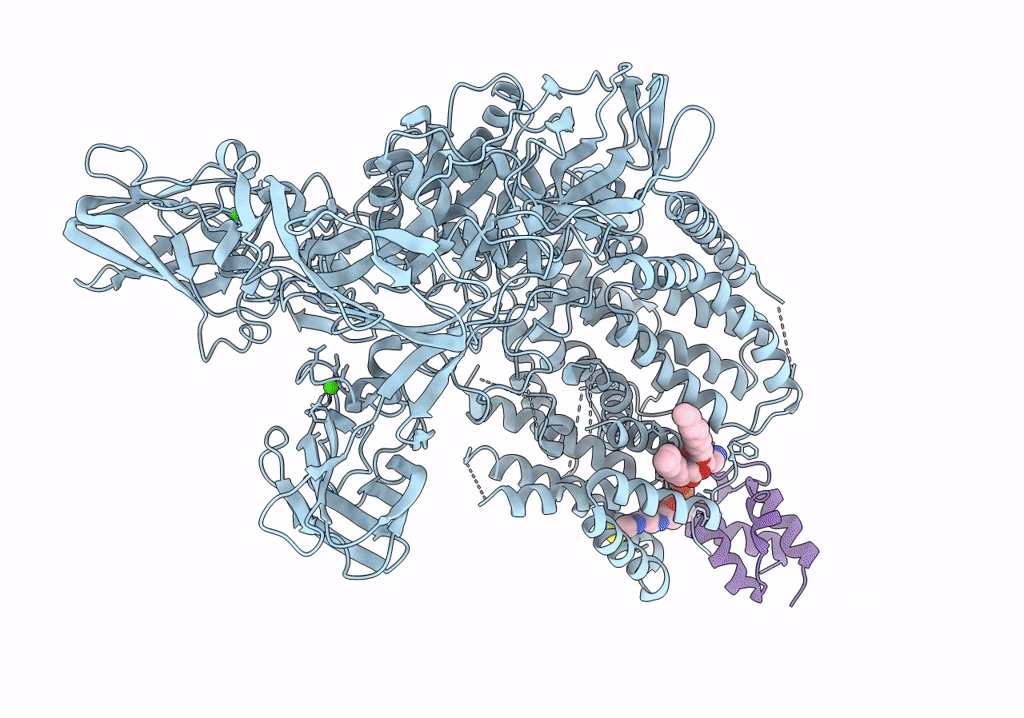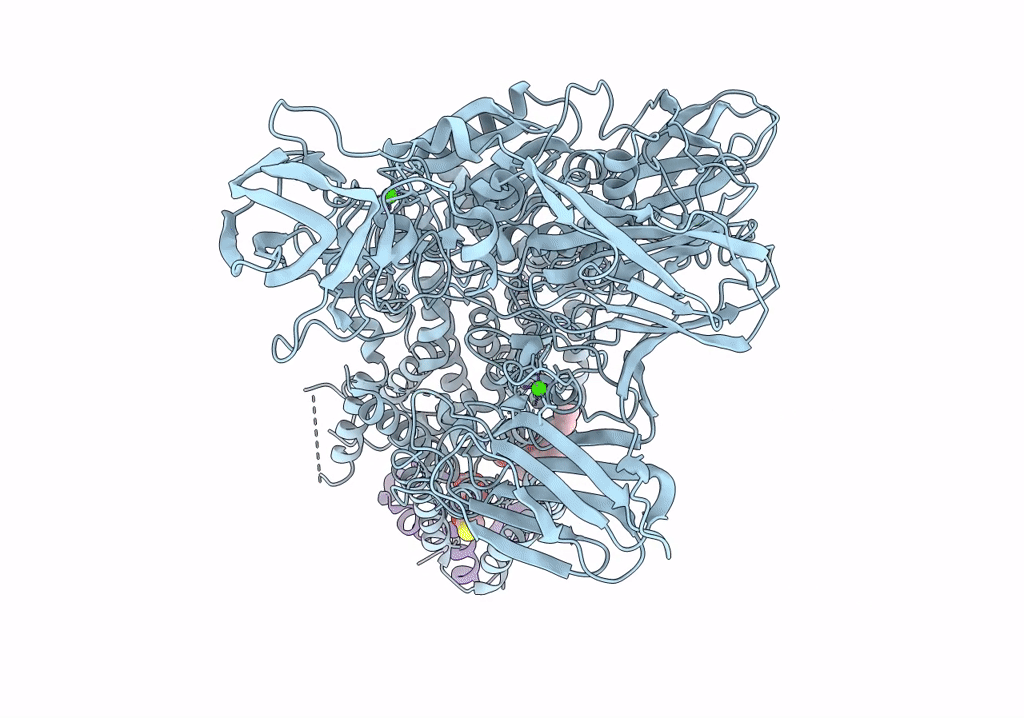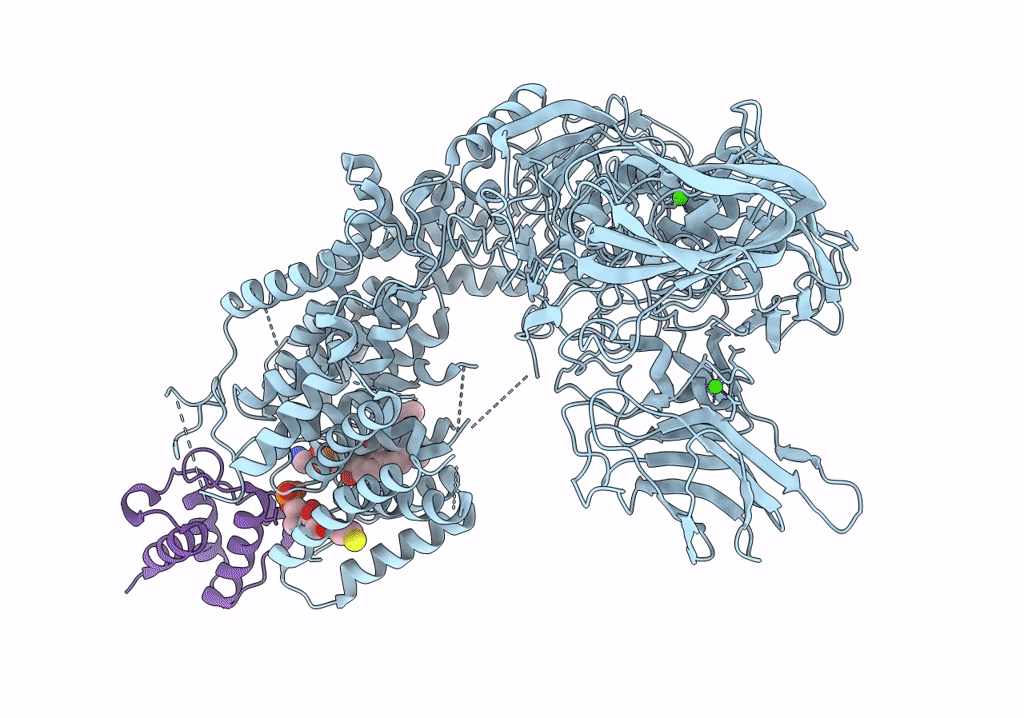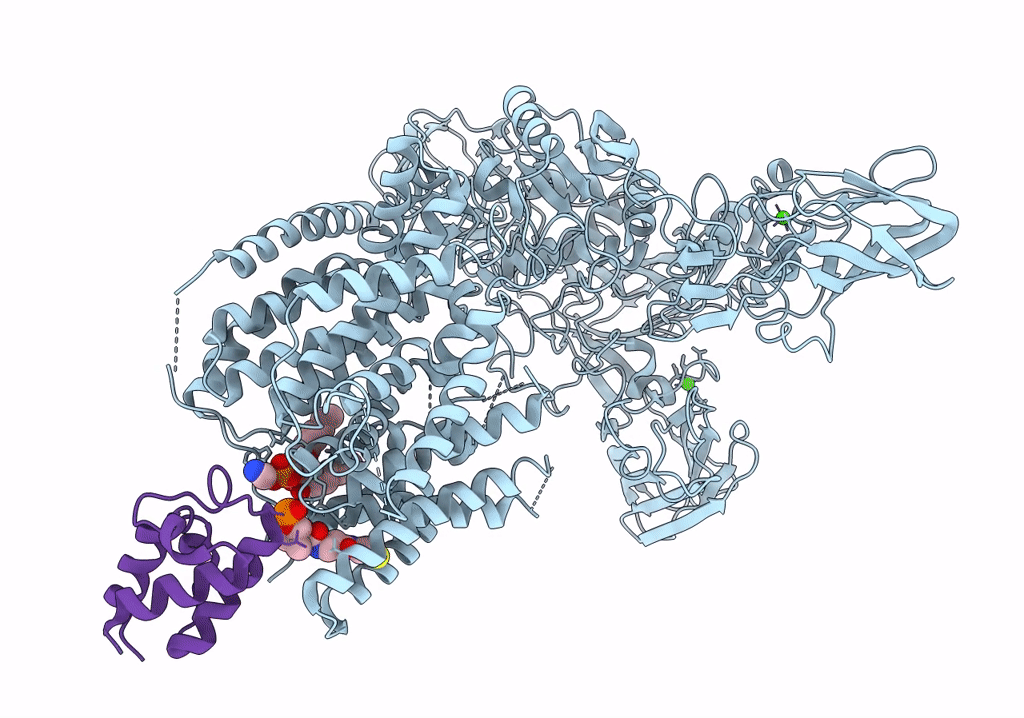
Single-Particle Cryo-EM Structure of Arabinofuranosyltransferase AftD from Mycobacteria
Biological Source:
Source Organism(s):
Mycobacteroides abscessus subsp. abscessus (Taxon ID: 1185650)
Escherichia coli (strain K12) (Taxon ID: 83333)
Escherichia coli (strain K12) (Taxon ID: 83333)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.90 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


