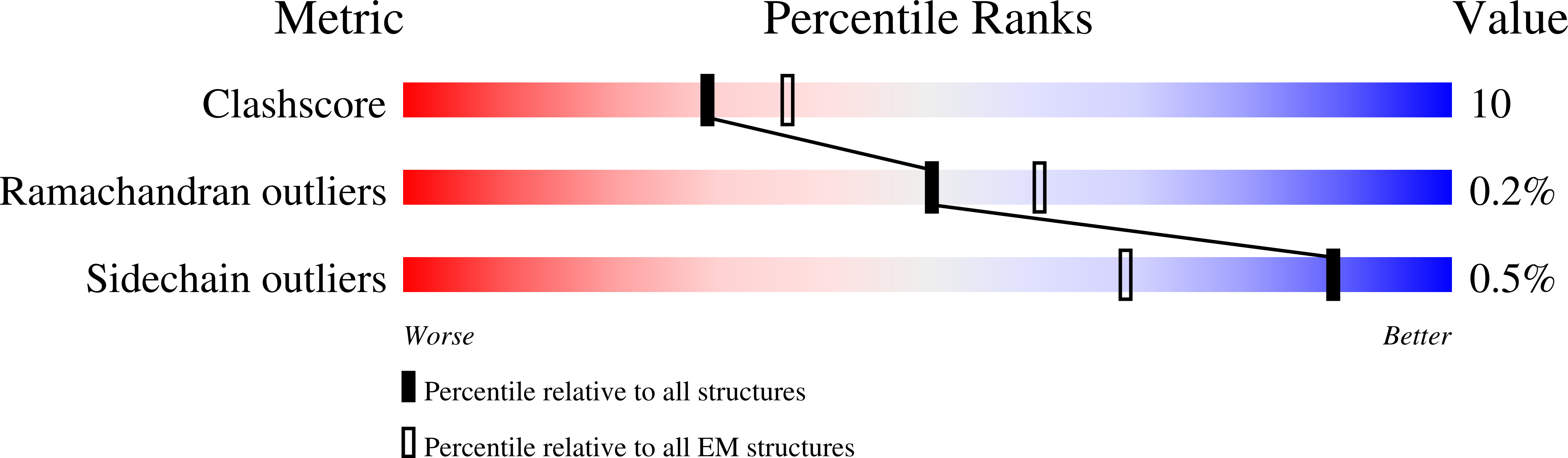
Deposition Date
2020-03-16
Release Date
2021-05-26
Last Version Date
2024-05-29
Entry Detail
PDB ID:
6W6E
Keywords:
Title:
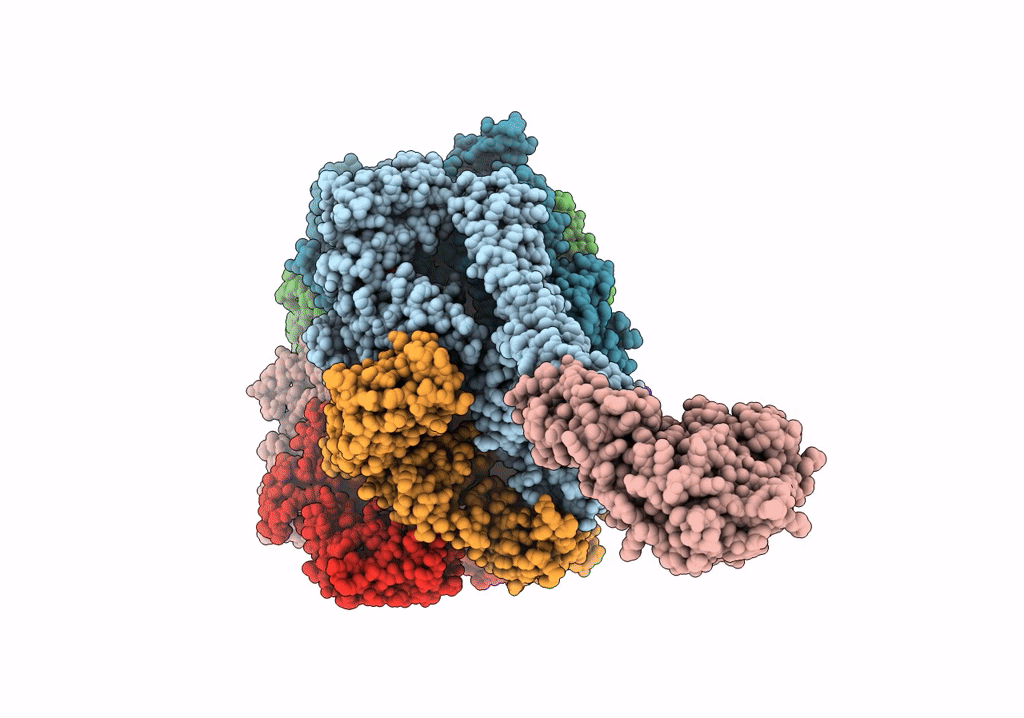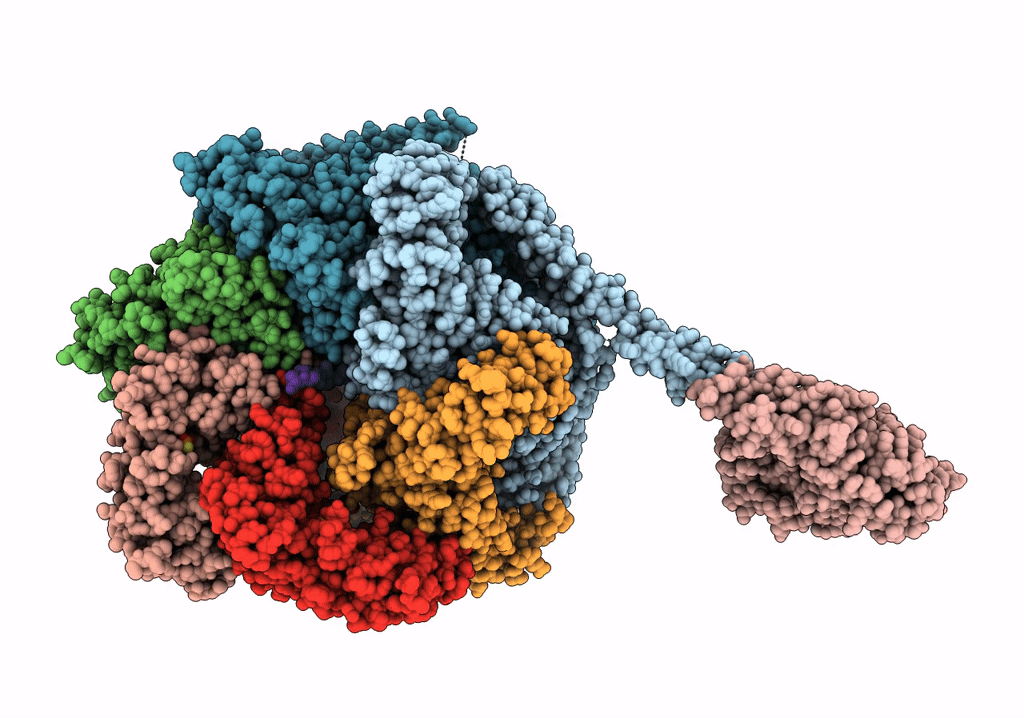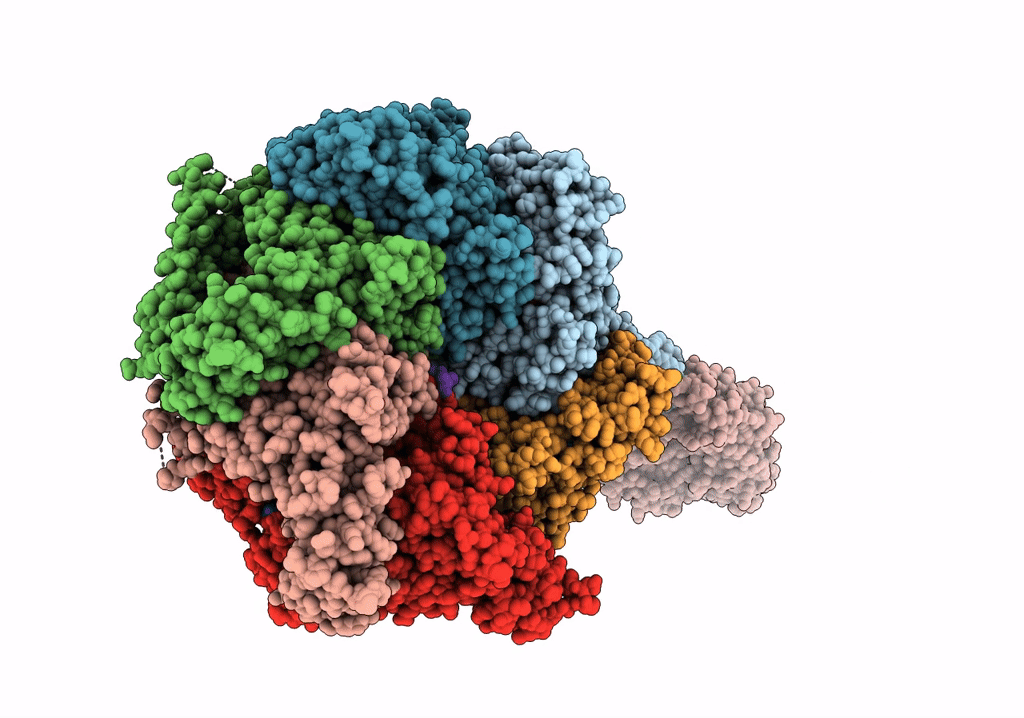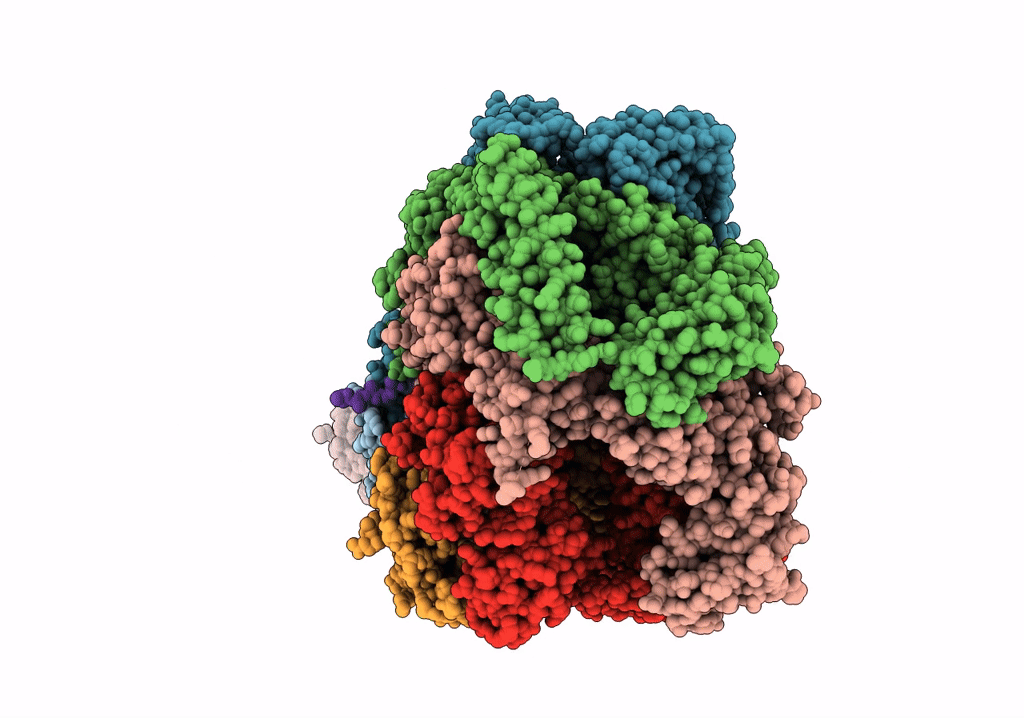
The Mycobacterium tuberculosis ClpB disaggregase hexamer structure with a locally refined ClpB middle domain and a DnaK nucleotide binding domain
Biological Source:
Source Organism:
Mycobacterium tuberculosis (Taxon ID: 1773)
Host Organism:
Method Details:
Experimental Method:
Resolution:
3.70 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE