Deposition Date
2020-03-04
Release Date
2020-07-29
Last Version Date
2024-10-23
Entry Detail
PDB ID:
6W1I
Keywords:
Title:
Re-interpretation of ppGpp (G4P) electron density in the deposited crystal structure of Xanthine phosphoribosyltransferase (XPRT) (1Y0B).
Biological Source:
Source Organism:
Bacillus subtilis (strain 168) (Taxon ID: 224308)
Host Organism:
Method Details:
Experimental Method:
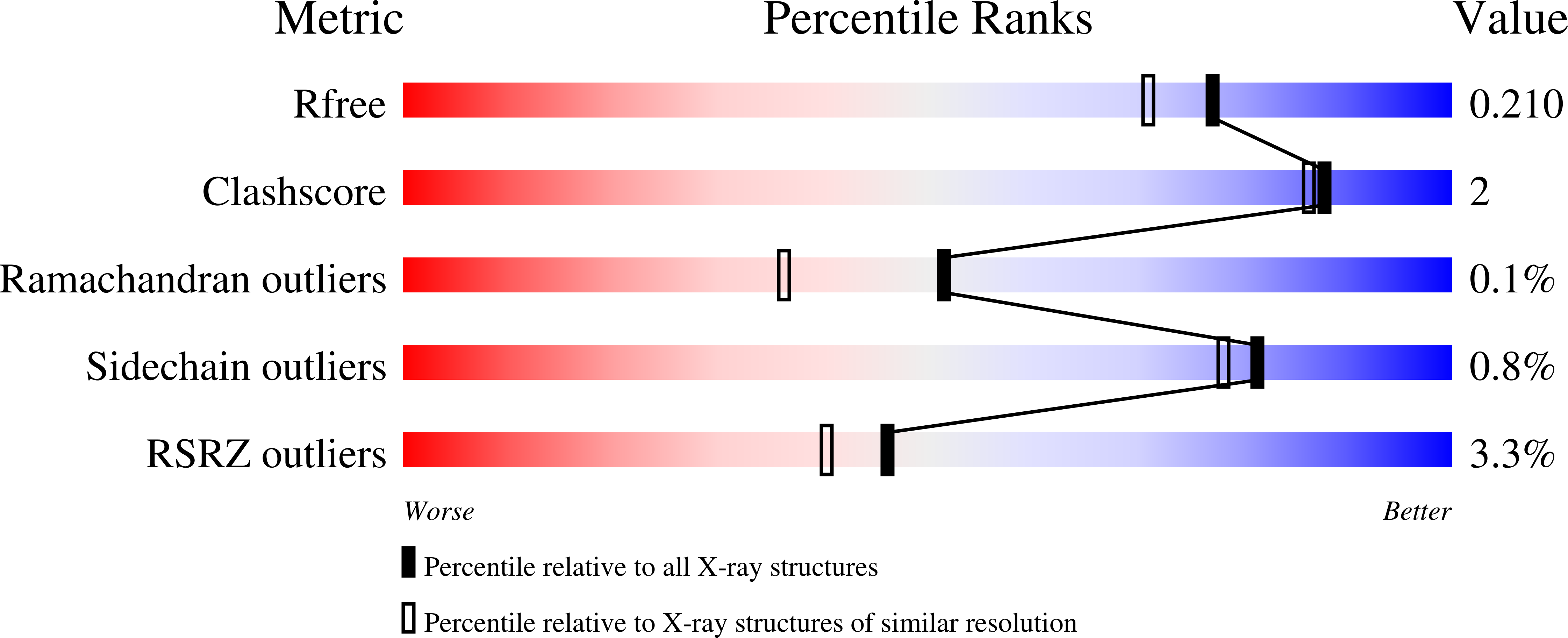
Resolution:
1.80 Å
R-Value Free:
0.20
R-Value Work:
0.15
R-Value Observed:
0.16
Space Group:
P 1 21 1