Deposition Date
2020-02-26
Release Date
2020-07-08
Last Version Date
2024-11-13
Entry Detail
PDB ID:
6VYD
Keywords:
Title:
Terpenoid Cyclase FgGS in Complex with Mg, Inorganic Pyrophosphate, and Benzyltriethylammonium cation
Biological Source:
Source Organism:
Host Organism:
Method Details:
Experimental Method:
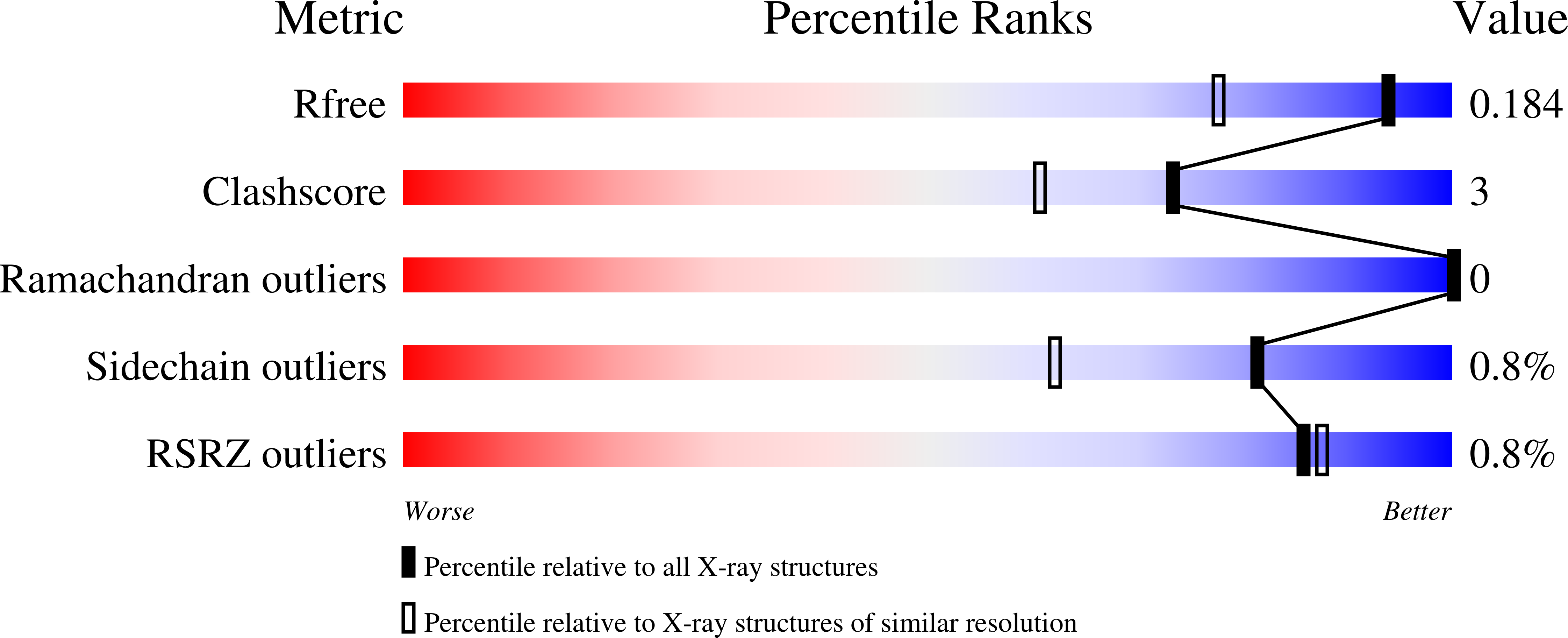
Resolution:
1.46 Å
R-Value Free:
0.18
R-Value Work:
0.14
R-Value Observed:
0.15
Space Group:
P 21 21 21