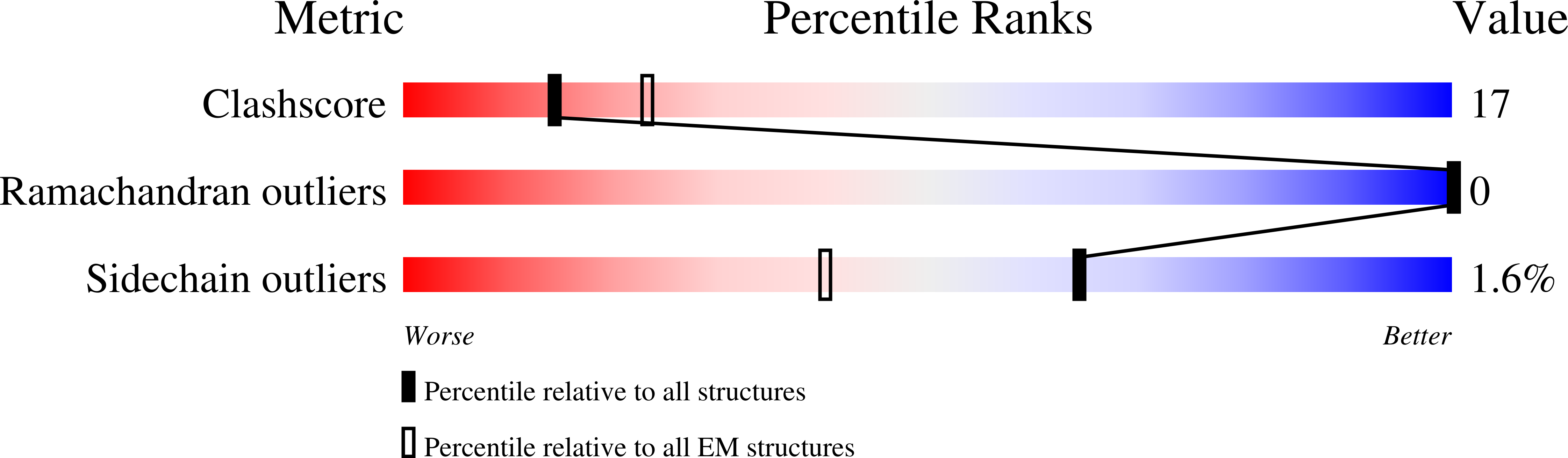Deposition Date
2020-02-04
Release Date
2020-04-22
Last Version Date
2024-11-20
Entry Detail
PDB ID:
6VPX
Keywords:
Title:
Nanodisc of full-length HIV-1 Envelope glycoprotein clone AMC011 in complex with one PGT151 Fab and three 10E8 Fabs
Biological Source:
Source Organism(s):
Human immunodeficiency virus 1 (Taxon ID: 11676)
Homo sapiens (Taxon ID: 9606)
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
5.00 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE