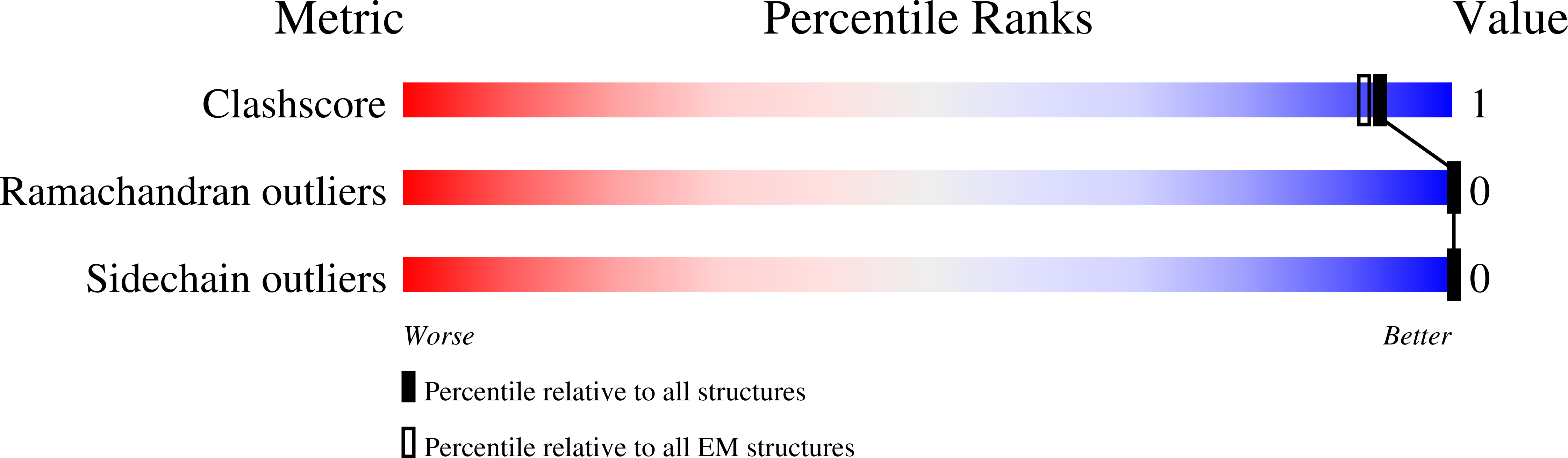
Deposition Date
2020-01-08
Release Date
2020-03-18
Last Version Date
2024-10-09
Entry Detail
PDB ID:
6VGQ
Keywords:
Title:
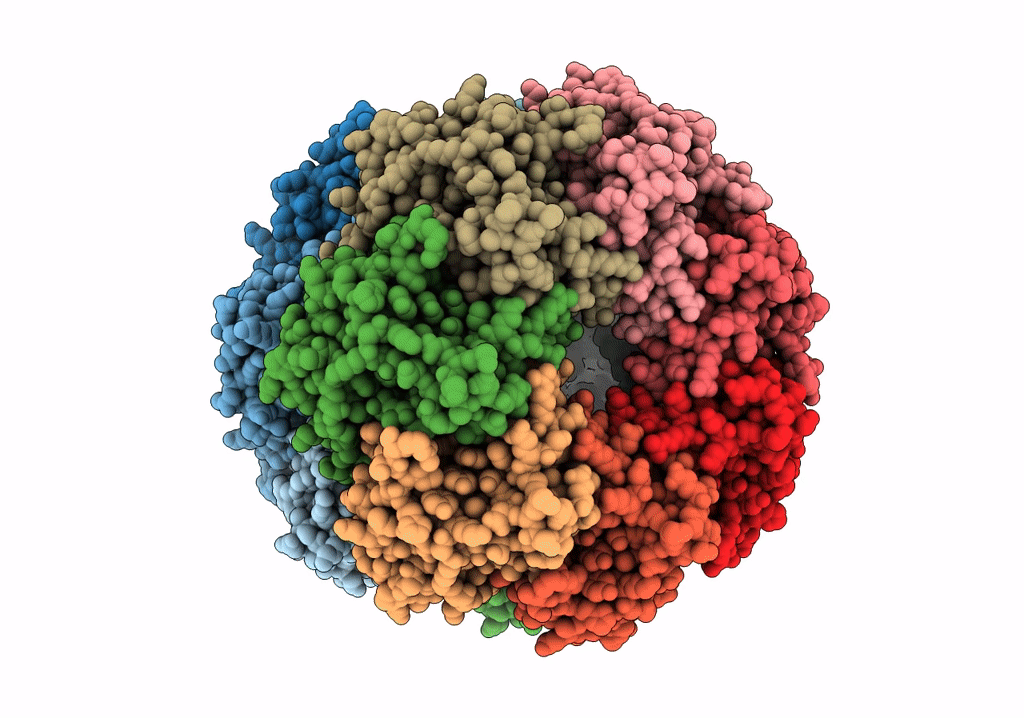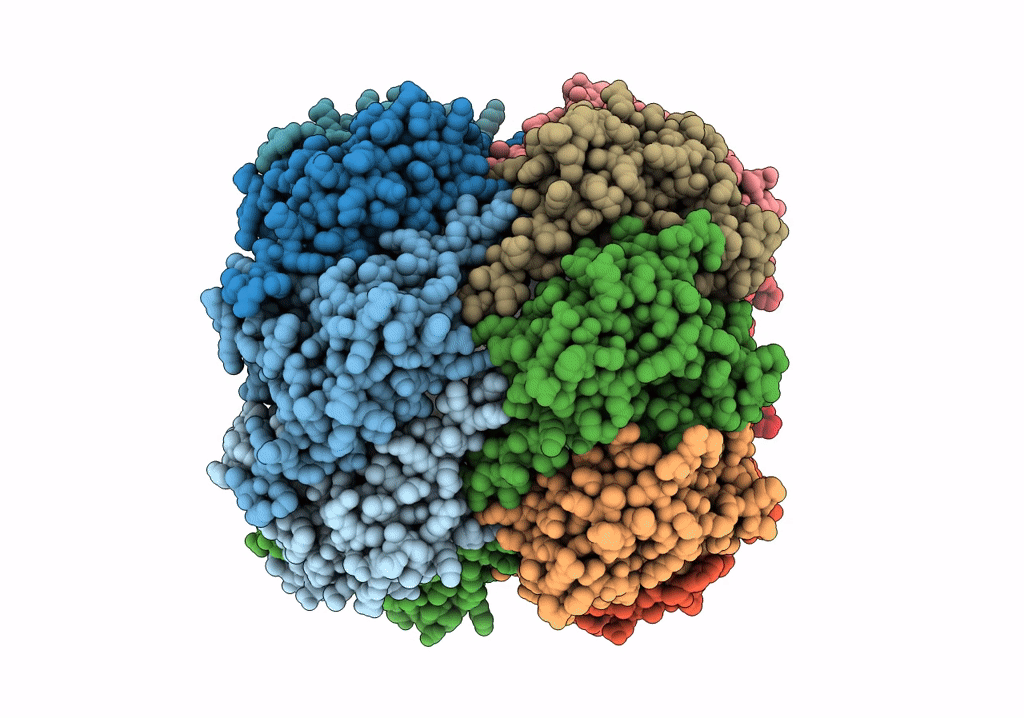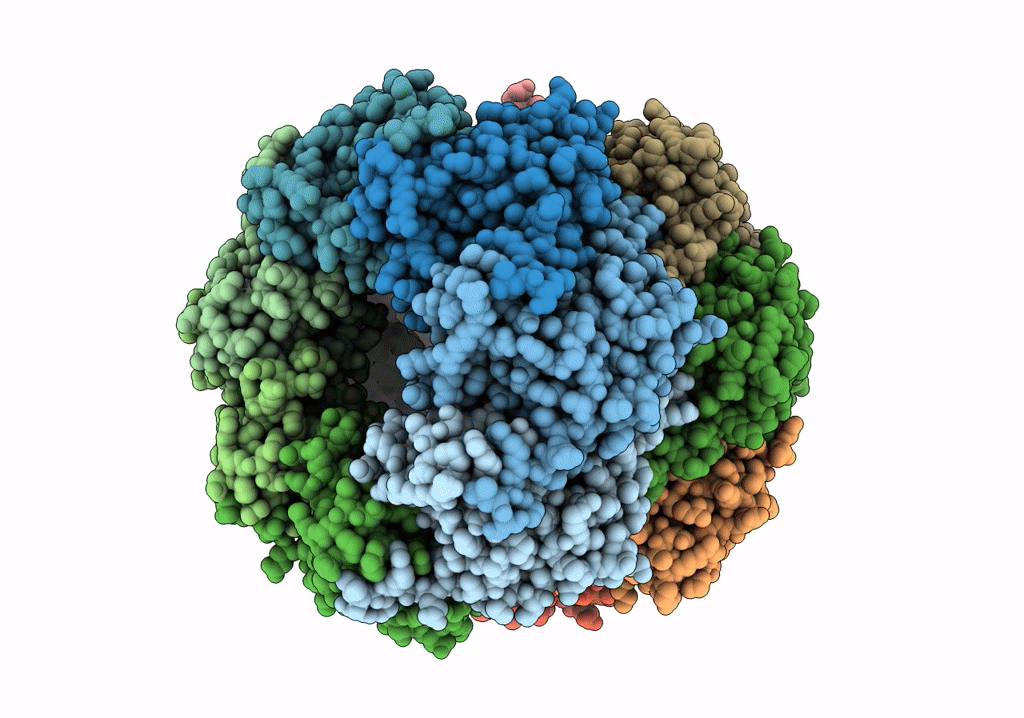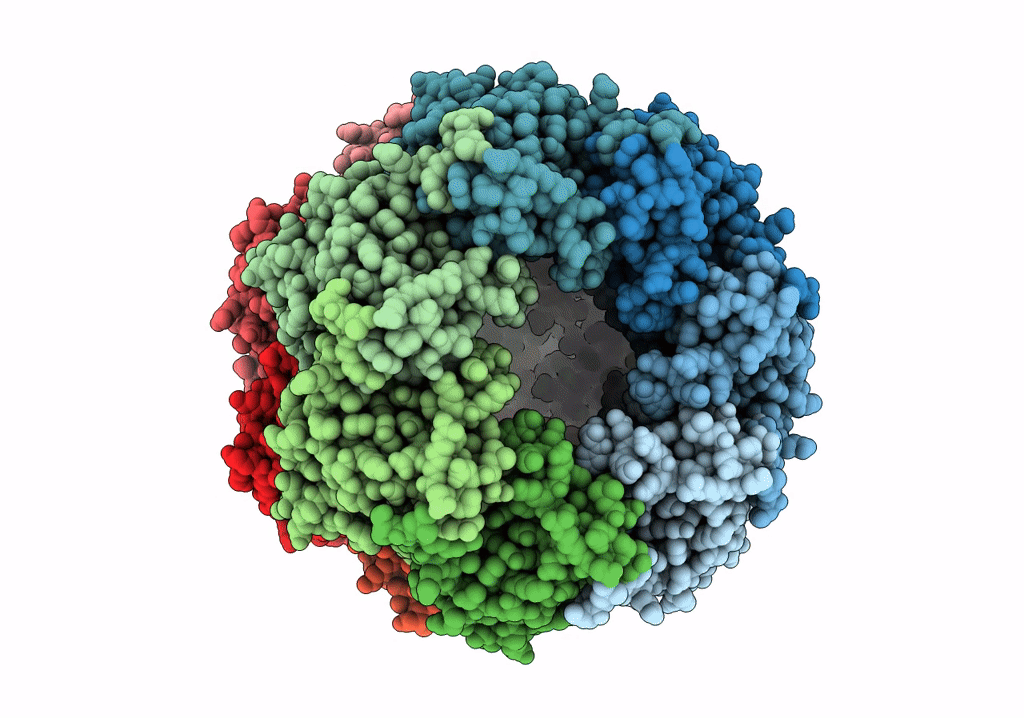
ClpP1P2 complex from M. tuberculosis with GLF-CMK bound to ClpP1
Biological Source:
Source Organism(s):
Mycobacterium tuberculosis (Taxon ID: 1773)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.50 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE