Deposition Date
2020-01-07
Release Date
2020-11-25
Last Version Date
2023-10-11
Entry Detail
PDB ID:
6VGD
Keywords:
Title:
Crystal structure of the DNA binding domain (DBD) of human FLI1 and the complex of the DBD of human Runx2 with core binding factor beta (Cbfb), in complex with 16mer DNA CAGAGGATGTGGCTTC
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
4.20 Å
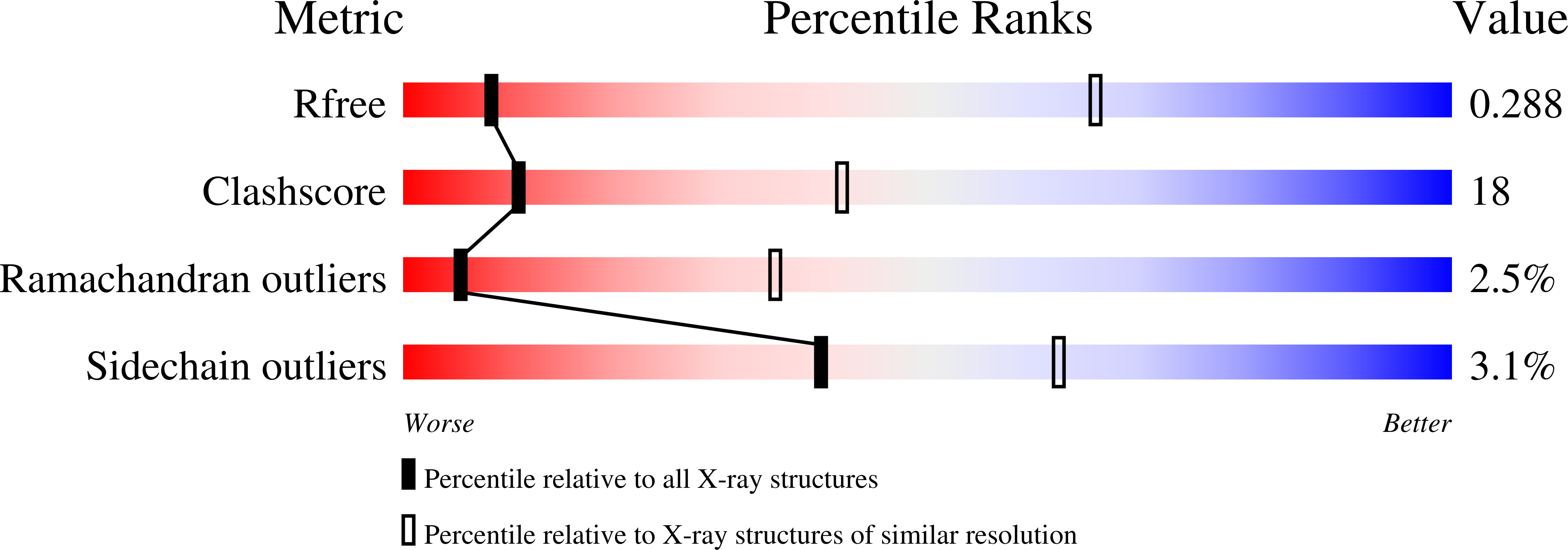
R-Value Free:
0.29
R-Value Work:
0.29
R-Value Observed:
0.29
Space Group:
P 62 2 2