Deposition Date
2019-11-26
Release Date
2020-10-21
Last Version Date
2023-11-15
Entry Detail
PDB ID:
6V3V
Keywords:
Title:
Assembly of VIQKI I456(beta-L-homoisoleucine)with human parainfluenza virus type 3 (HPIV3) fusion glycoprotein N-terminal heptad repeat domain
Biological Source:
Source Organism(s):
Method Details:
Experimental Method:
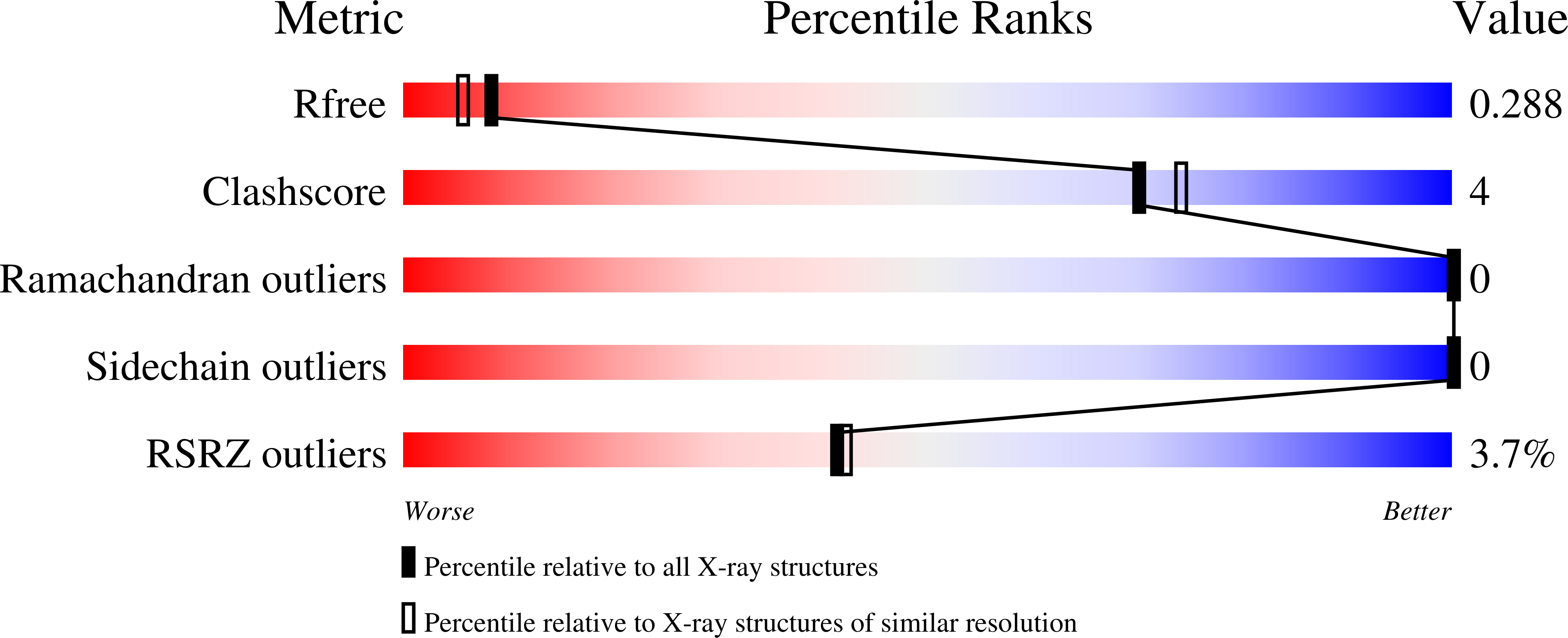
Resolution:
2.17 Å
R-Value Free:
0.28
R-Value Work:
0.24
R-Value Observed:
0.24
Space Group:
P 1 21 1