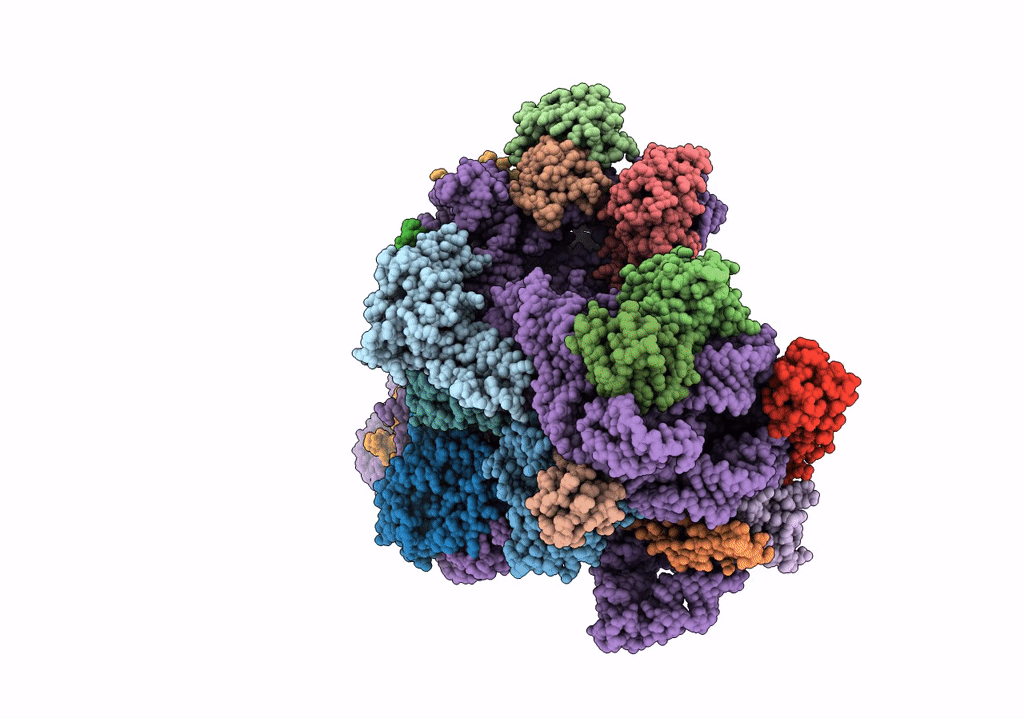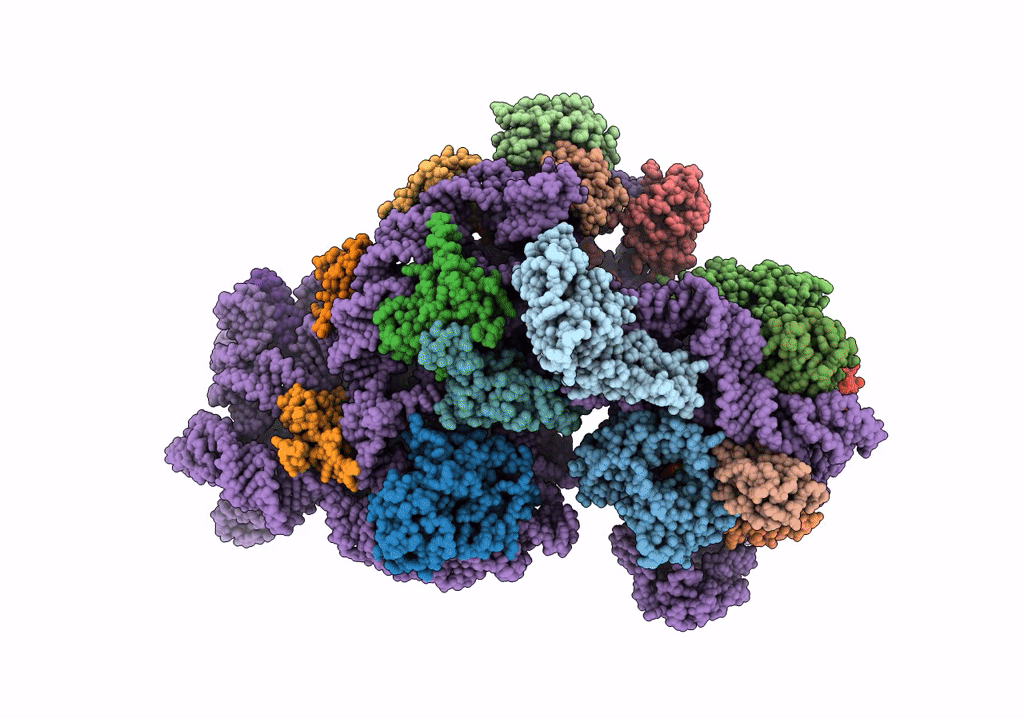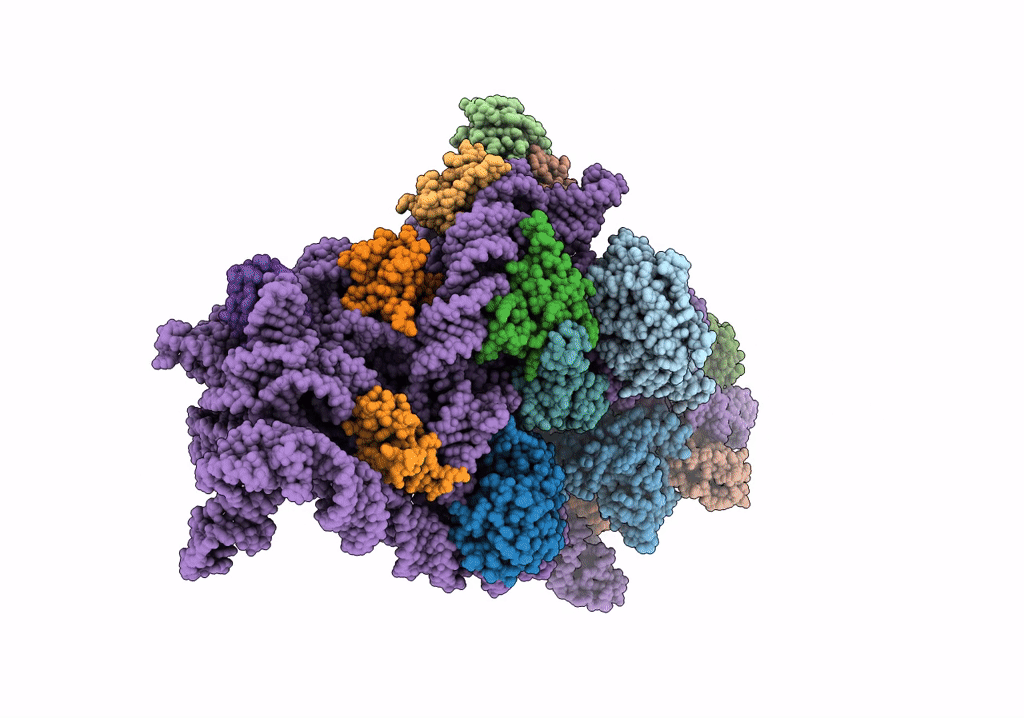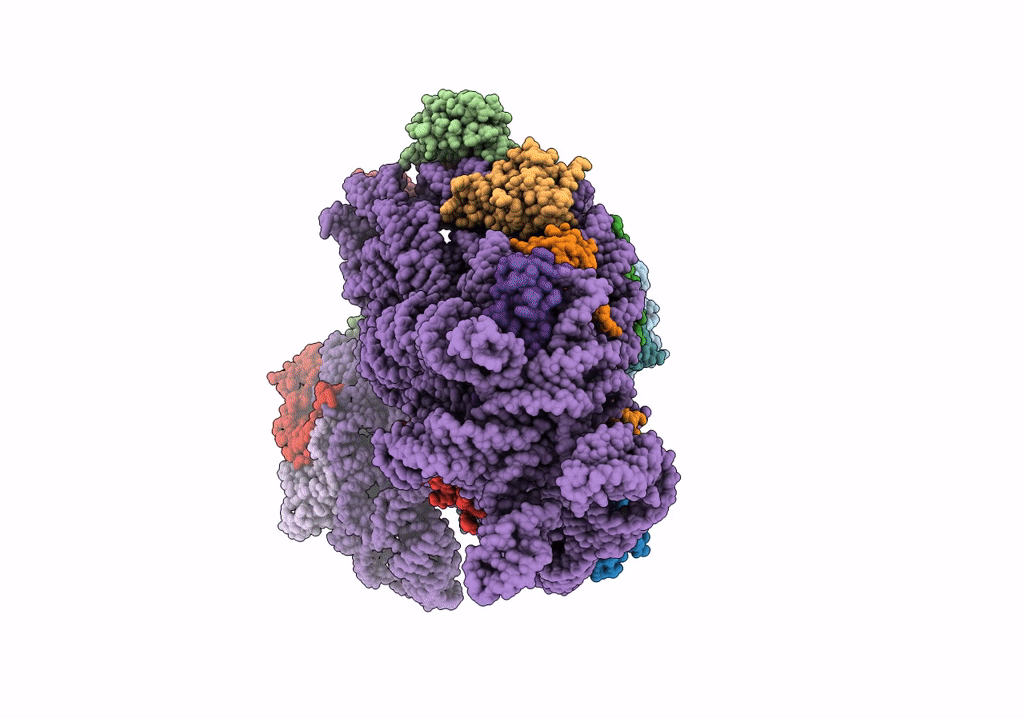
Deposition Date
2019-11-25
Release Date
2020-02-05
Last Version Date
2024-10-16
Entry Detail
PDB ID:
6V3E
Keywords:
Title:
Cryo-EM structure of the Acinetobacter baumannii Ribosome: 30S subunit
Biological Source:
Source Organism(s):
Acinetobacter baumannii (Taxon ID: 470)
Acinetobacter baumannii (strain AB0057) (Taxon ID: 480119)
Acinetobacter sp. ANC 5600 (Taxon ID: 1960940)
Acinetobacter sp. 263903-1 (Taxon ID: 1310678)
Acinetobacter venetianus (Taxon ID: 52133)
Acinetobacter baumannii (strain AB0057) (Taxon ID: 480119)
Acinetobacter sp. ANC 5600 (Taxon ID: 1960940)
Acinetobacter sp. 263903-1 (Taxon ID: 1310678)
Acinetobacter venetianus (Taxon ID: 52133)
Method Details:
Experimental Method:
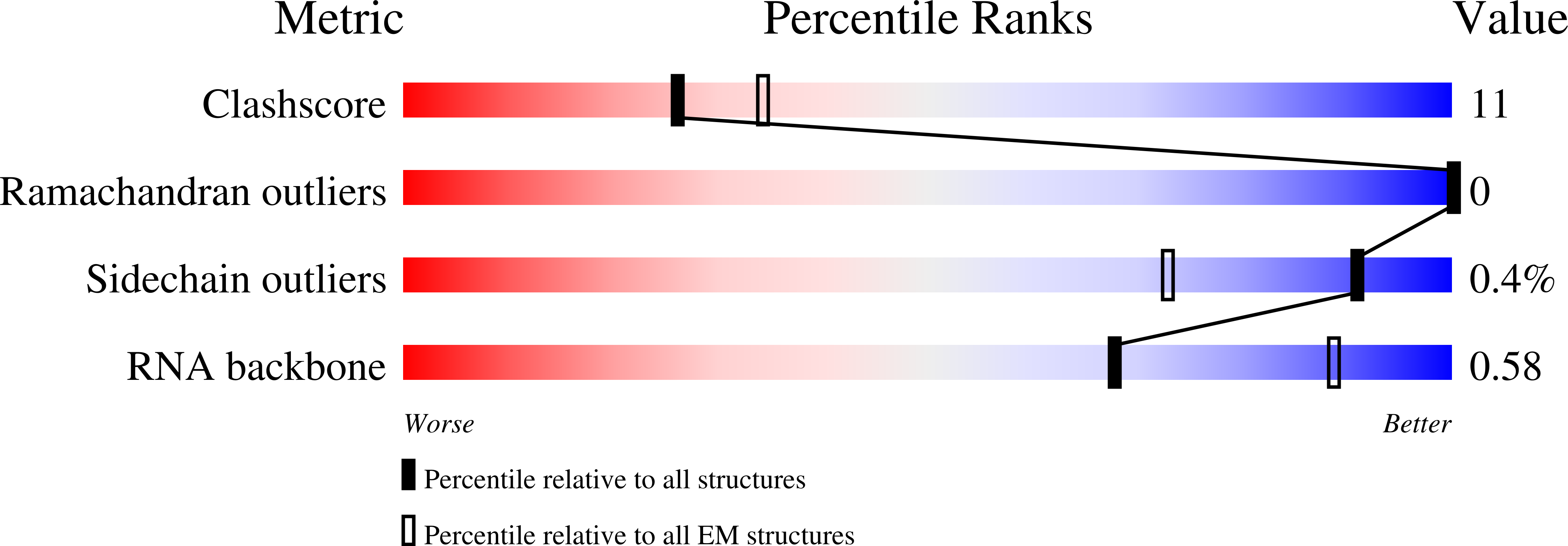
Resolution:
4.40 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE