Deposition Date
2019-11-13
Release Date
2020-12-02
Last Version Date
2023-10-11
Entry Detail
PDB ID:
6UYK
Keywords:
Title:
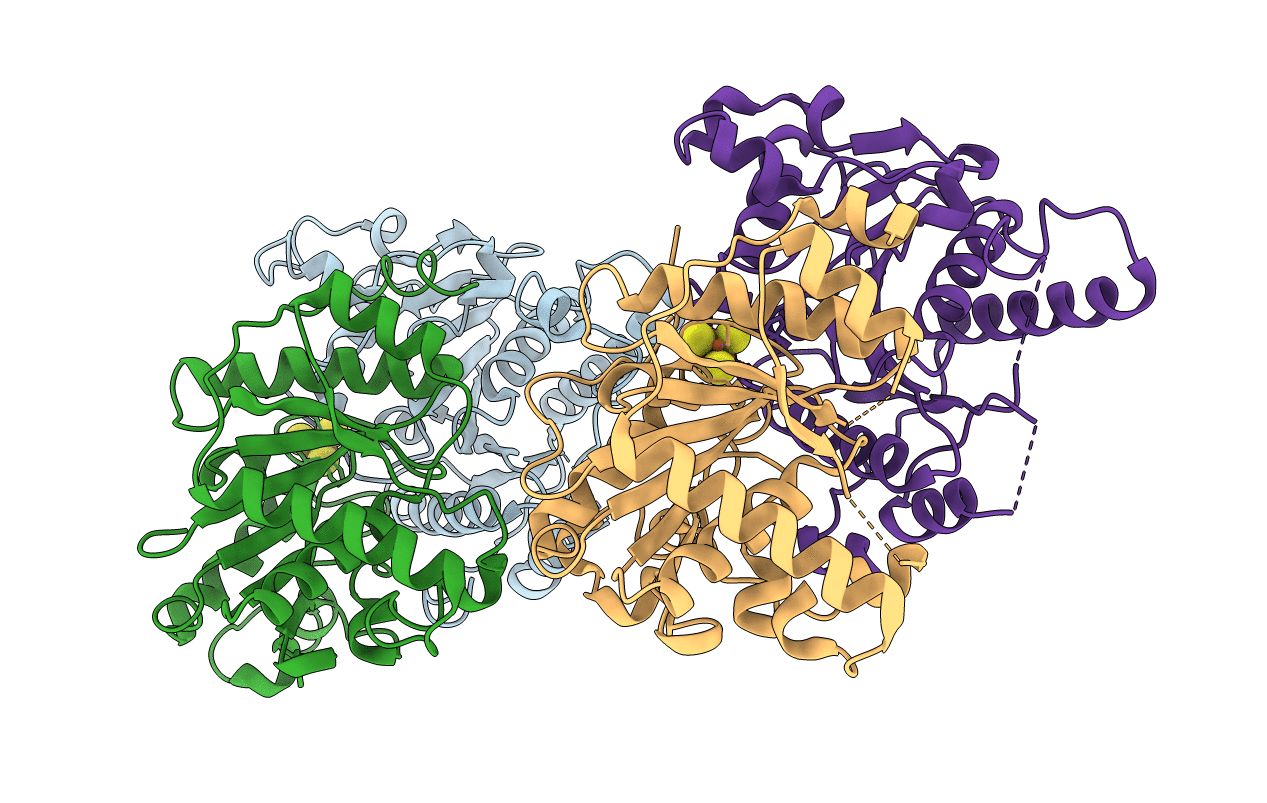
Dark-operative protochlorophyllide oxidoreductase in the nucleotide-free form.
Biological Source:
Source Organism:
Rhodobacter sphaeroides (Taxon ID: 1063)
Host Organism:
Method Details:
Experimental Method:
Resolution:
2.60 Å
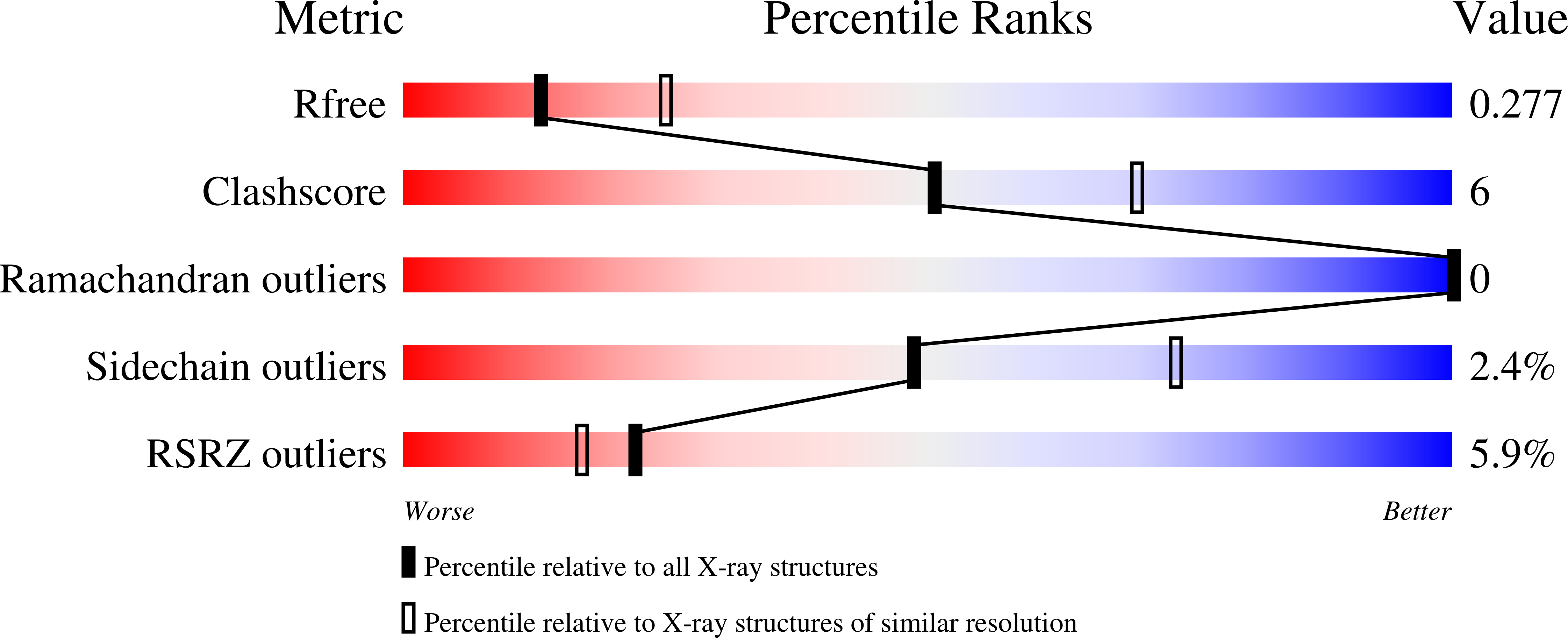
R-Value Free:
0.27
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
I 1 2 1