Deposition Date
2019-09-11
Release Date
2020-05-20
Last Version Date
2024-03-13
Entry Detail
PDB ID:
6UBB
Keywords:
Title:
Crystal structure of a GH128 (subgroup VI) exo-beta-1,3-glucanase from Aureobasidium namibiae (AnGH128_VI) with laminaribiose at the surface-binding site
Biological Source:
Source Organism(s):
Aureobasidium namibiae CBS 147.97 (Taxon ID: 1043004)
Expression System(s):
Method Details:
Experimental Method:
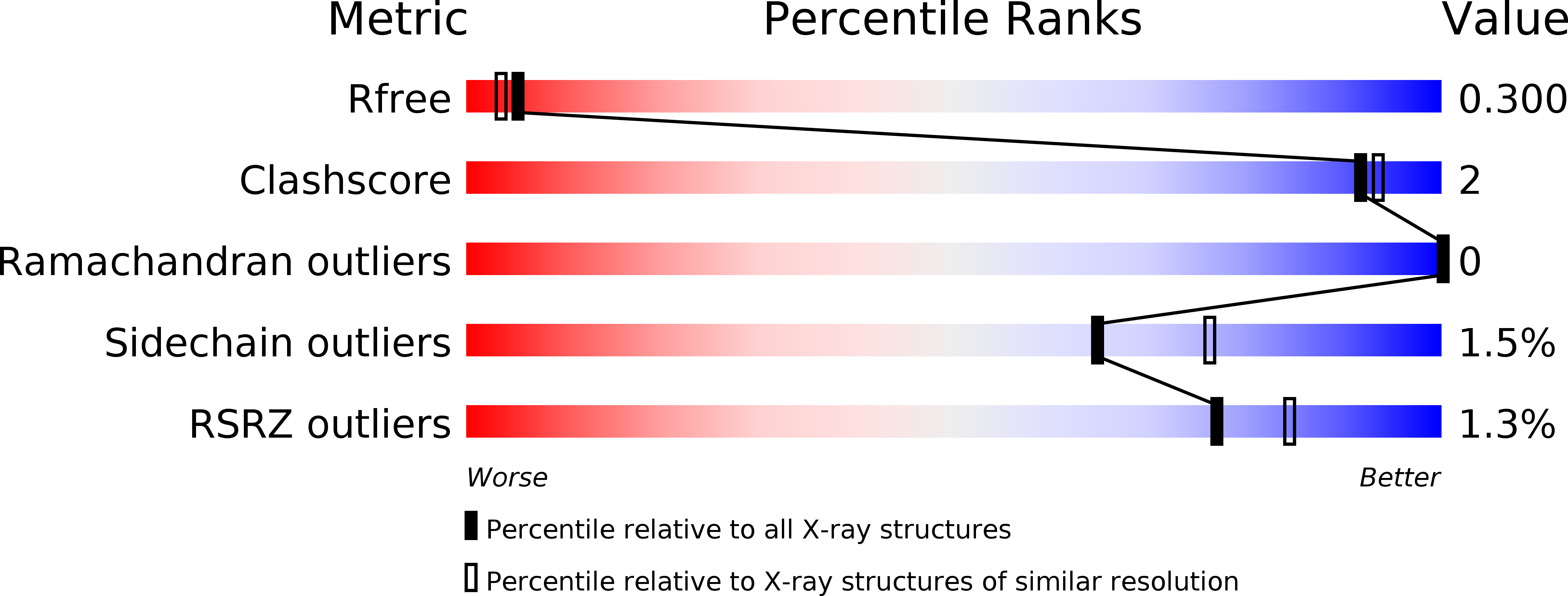
Resolution:
2.35 Å
R-Value Free:
0.30
R-Value Work:
0.26
Space Group:
P 21 21 21