Deposition Date
2019-08-23
Release Date
2019-09-18
Last Version Date
2023-11-15
Entry Detail
PDB ID:
6U48
Keywords:
Title:
E. coli 50S with phazolicin (PHZ) bound in exit tunnel
Biological Source:
Source Organism:
Escherichia coli (Taxon ID: 562)
Rhizobium sp. Pop5 (Taxon ID: 1223565)
Rhizobium sp. Pop5 (Taxon ID: 1223565)
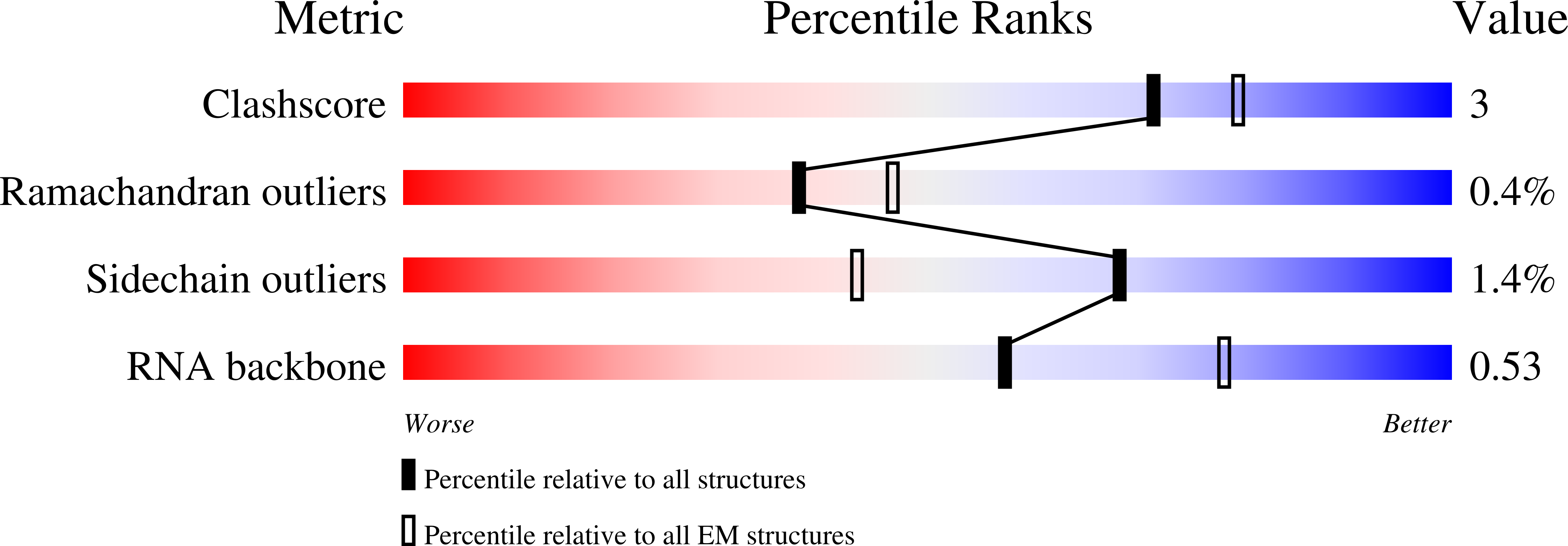
Method Details:
Experimental Method:
Resolution:
2.87 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE