Deposition Date
2019-08-14
Release Date
2020-09-23
Last Version Date
2023-10-11
Entry Detail
PDB ID:
6U0O
Keywords:
Title:
Crystal structure of a peptidoglycan release complex, SagB-SpdC, in lipidic cubic phase
Biological Source:
Source Organism:
Staphylococcus aureus (strain bovine RF122 / ET3-1) (Taxon ID: 273036)
Staphylococcus aureus (strain NCTC 8325) (Taxon ID: 93061)
Synthetic construct (Taxon ID: 32630)
Staphylococcus aureus (strain NCTC 8325) (Taxon ID: 93061)
Synthetic construct (Taxon ID: 32630)
Host Organism:
Method Details:
Experimental Method:
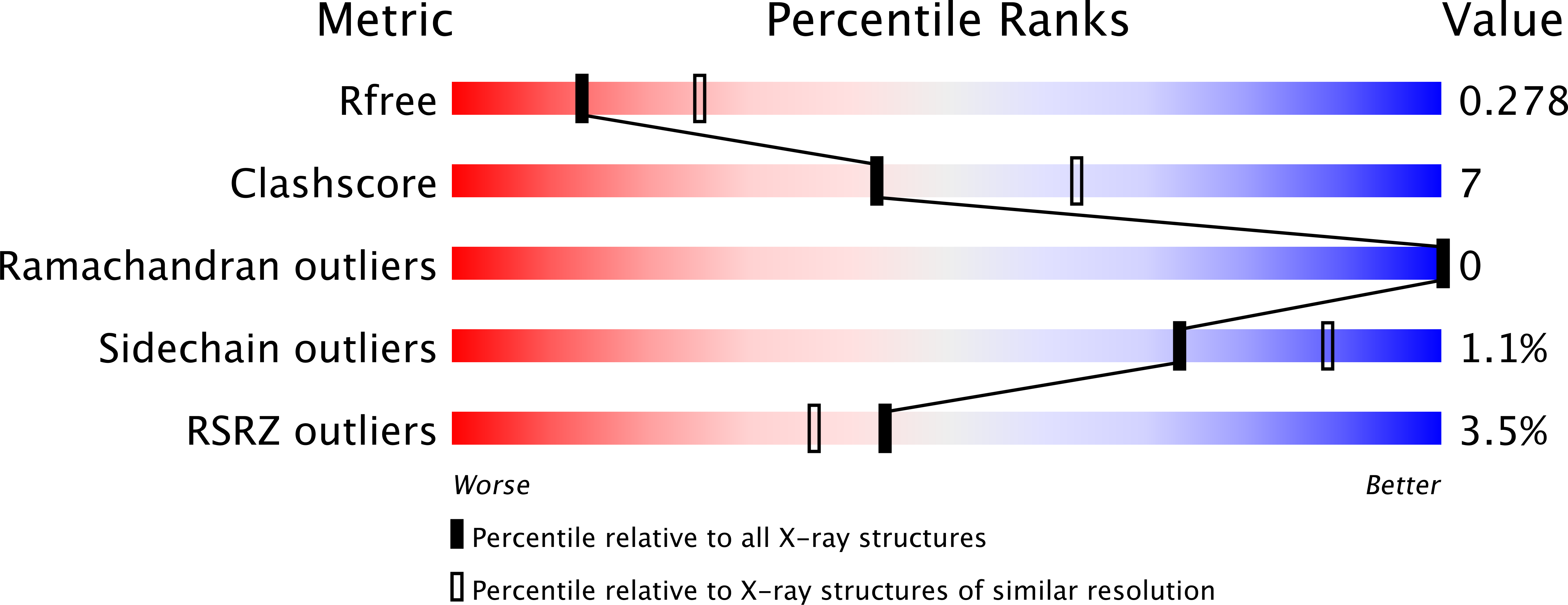
Resolution:
2.60 Å
R-Value Free:
0.27
R-Value Work:
0.25
R-Value Observed:
0.25
Space Group:
C 1 2 1