Deposition Date
2020-01-14
Release Date
2020-10-21
Last Version Date
2024-10-16
Entry Detail
PDB ID:
6TXO
Keywords:
Title:
Crystal structure of the haemagglutinin mutant (Gln226Leu, Del228) from an H10N7 seal influenza virus isolated in Germany in complex with avian receptor analogue 3'-SLN
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
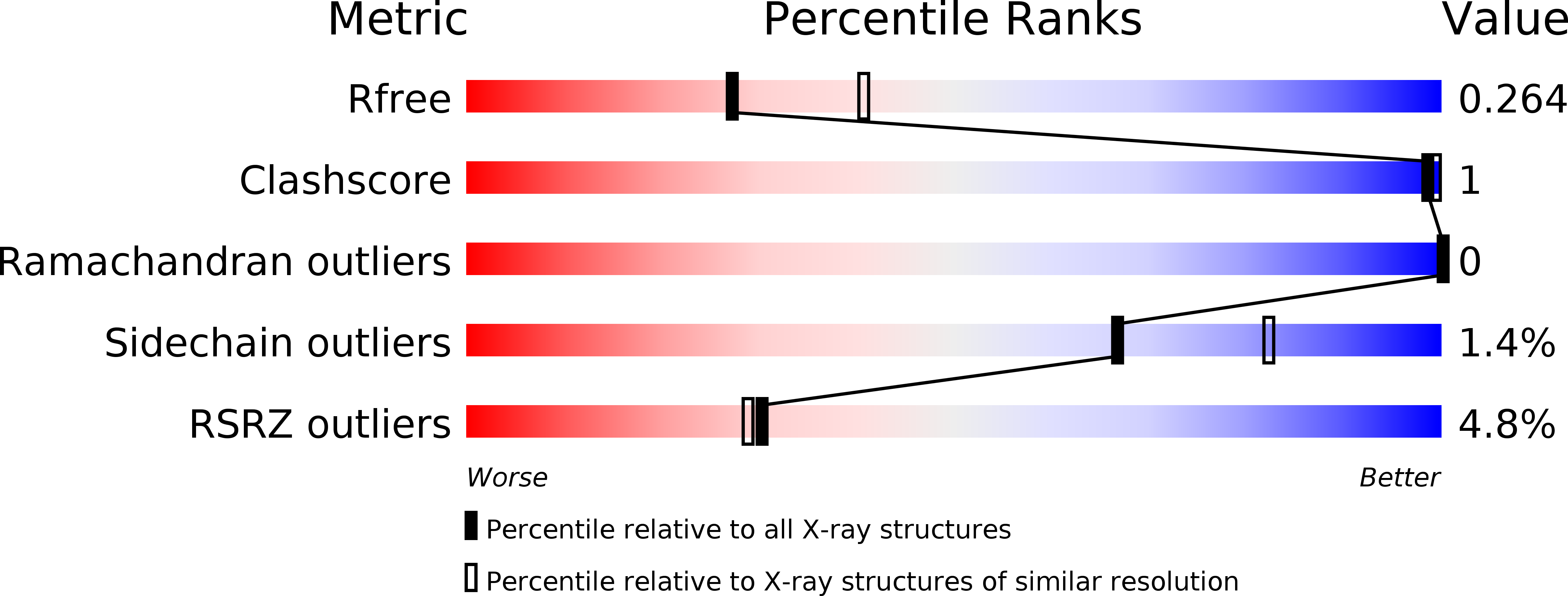
Resolution:
2.40 Å
R-Value Free:
0.27
R-Value Work:
0.24
R-Value Observed:
0.25
Space Group:
P 1 21 1