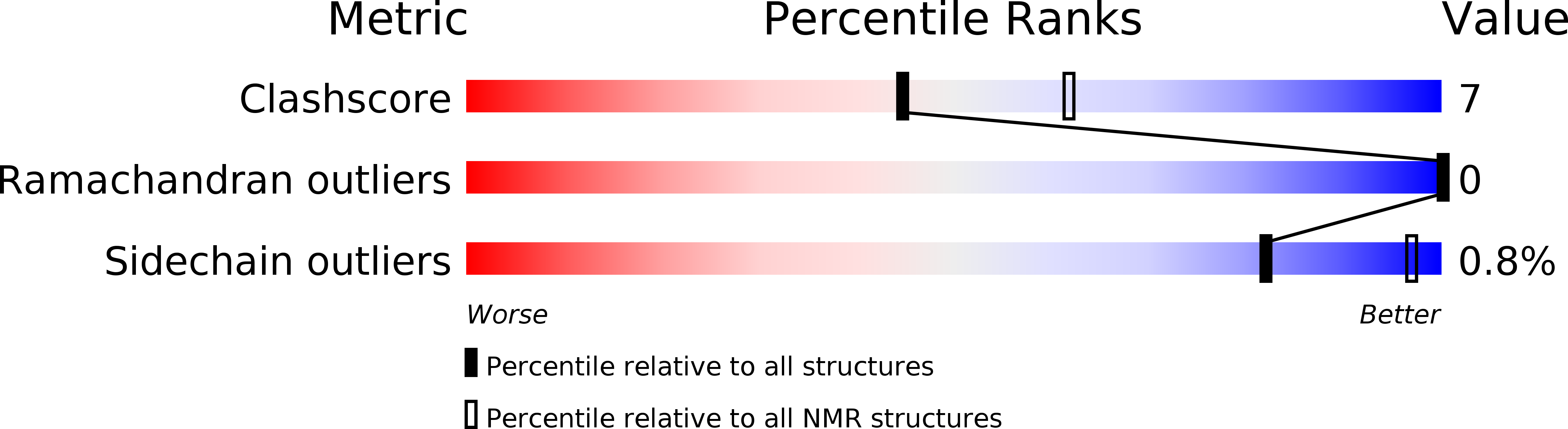
Deposition Date
2020-01-13
Release Date
2020-07-22
Last Version Date
2024-06-19
Entry Detail
PDB ID:
6TWG
Keywords:
Title:
Solution structure of antimicrobial peptide, crabrolin Plus in the presence of Lipopolysaccharide
Biological Source:
Source Organism(s):
Vespa crabro (Taxon ID: 7445)
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
10
Selection Criteria:
target function