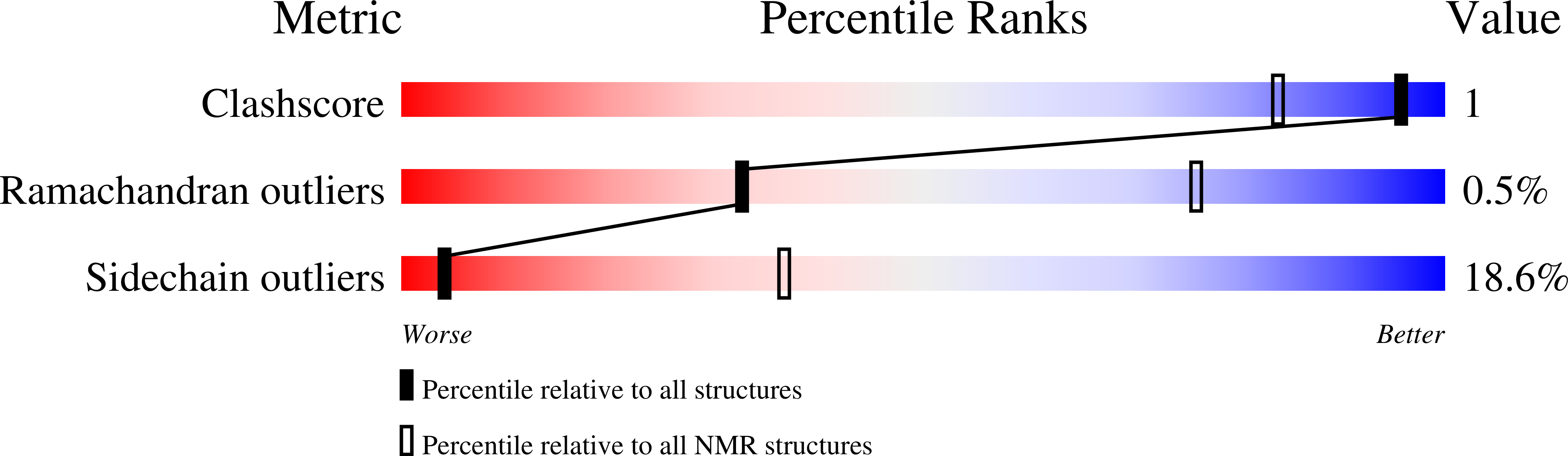
Deposition Date
2019-12-19
Release Date
2020-08-12
Last Version Date
2024-06-19
Entry Detail
PDB ID:
6TRP
Keywords:
Title:
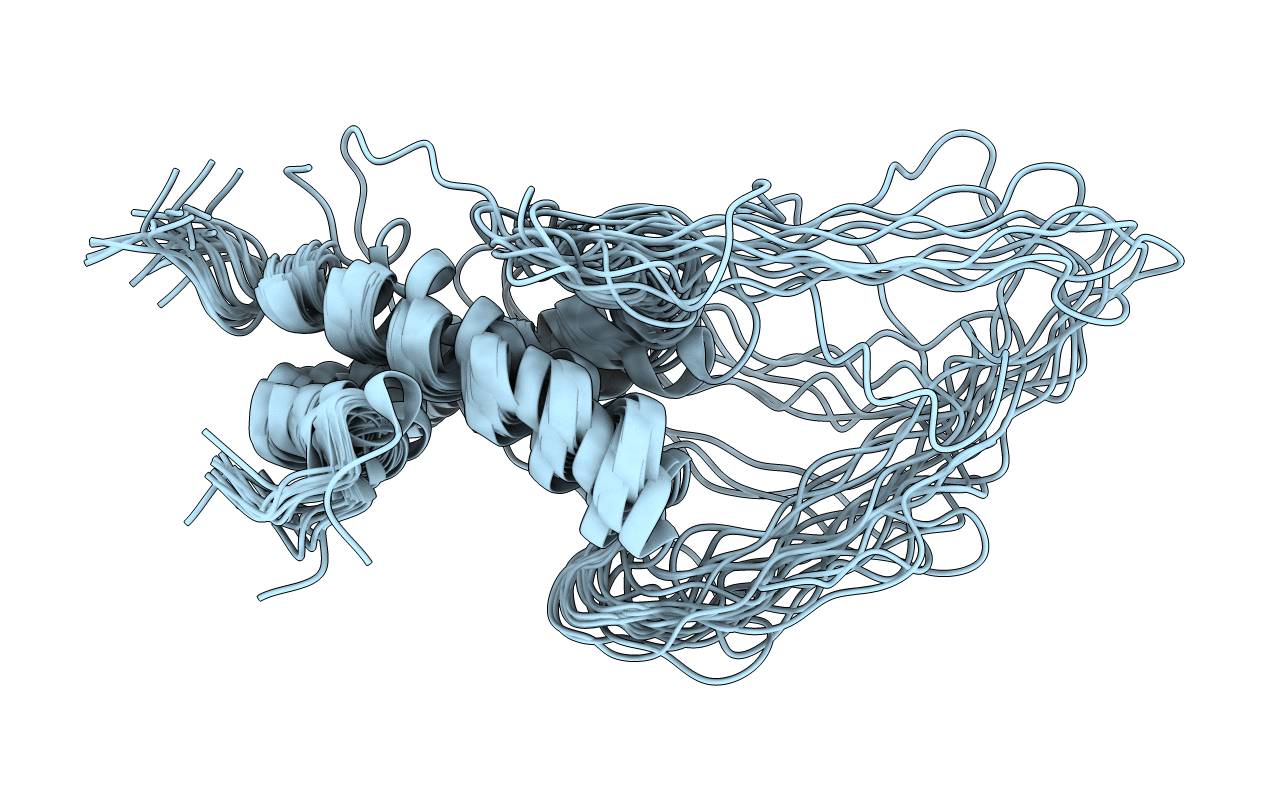
Solution Structure of Docking Domain Complex of Pax NRPS: PaxC NDD - PaxB CDD
Biological Source:
Source Organism(s):
Xenorhabdus bovienii SS-2004 (Taxon ID: 406818)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
20
Conformers Submitted:
20
Selection Criteria:
structures with the least restraint violations