Deposition Date
2019-11-28
Release Date
2020-05-27
Last Version Date
2024-10-16
Entry Detail
PDB ID:
6TK4
Keywords:
Title:
Femtosecond to millisecond structural changes in a light-driven sodium pump: 1ns+16ns structure of KR2 with extrapolated, light and dark datasets
Biological Source:
Source Organism(s):
Dokdonia eikasta (Taxon ID: 308116)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.25 Å
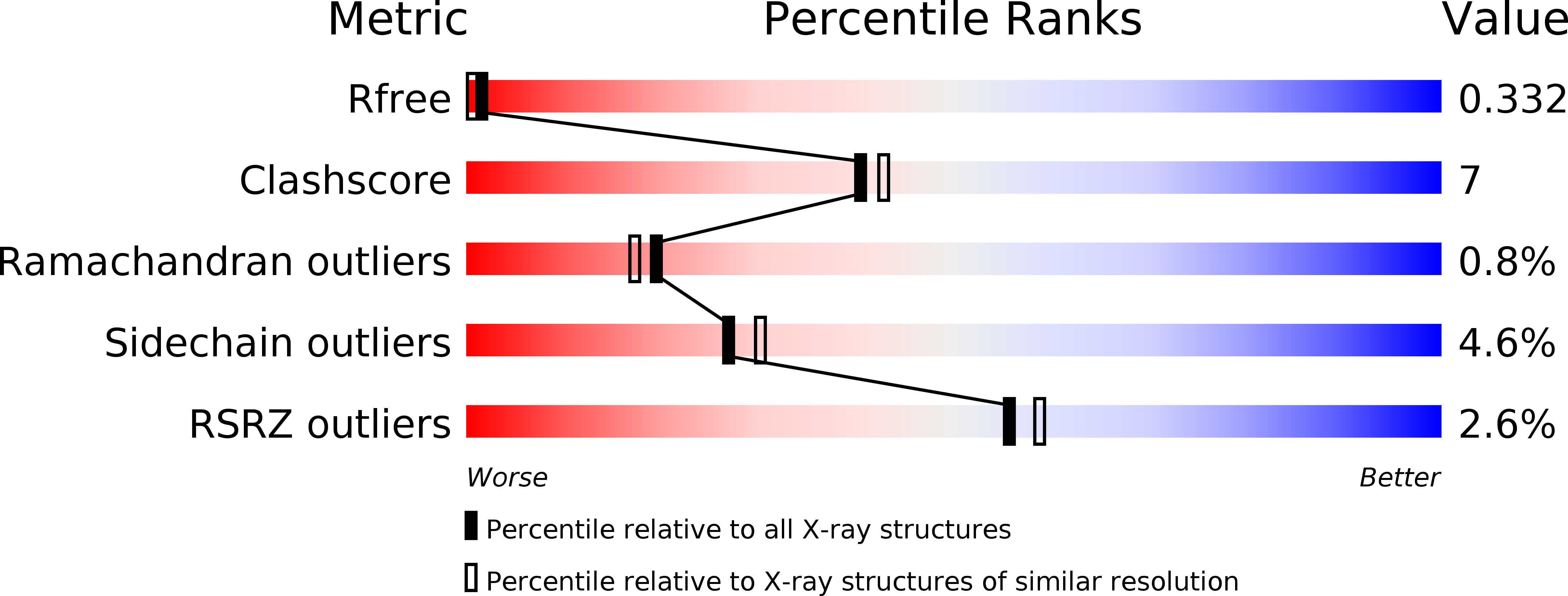
R-Value Free:
0.33
R-Value Work:
0.26
R-Value Observed:
0.26
Space Group:
I 2 2 2