Deposition Date
2019-11-20
Release Date
2020-12-09
Last Version Date
2024-05-22
Entry Detail
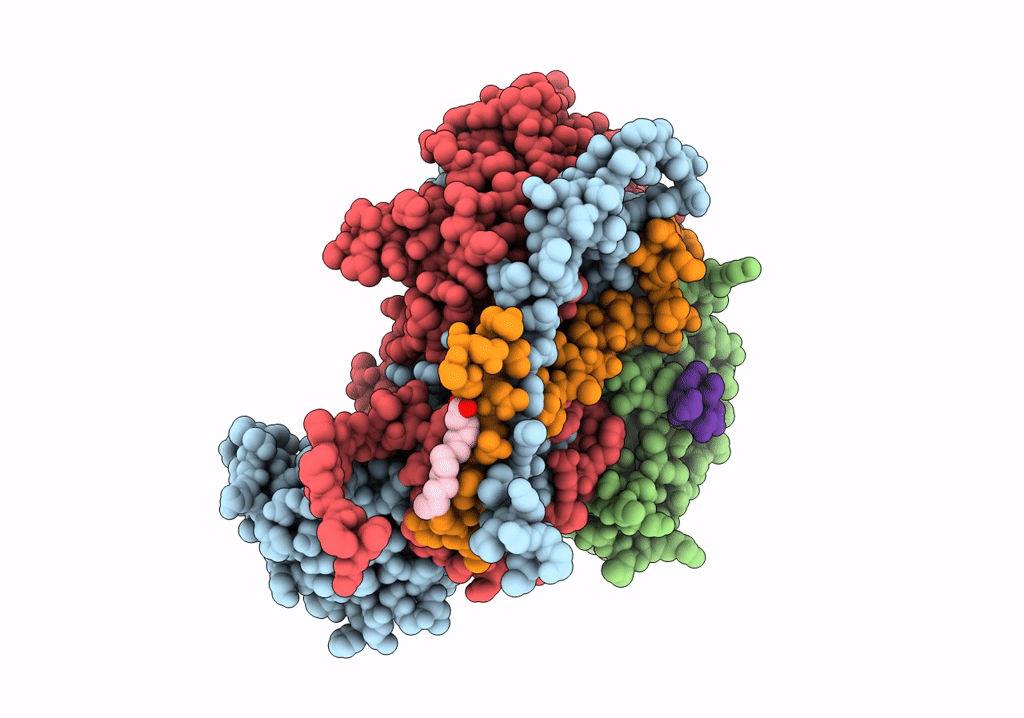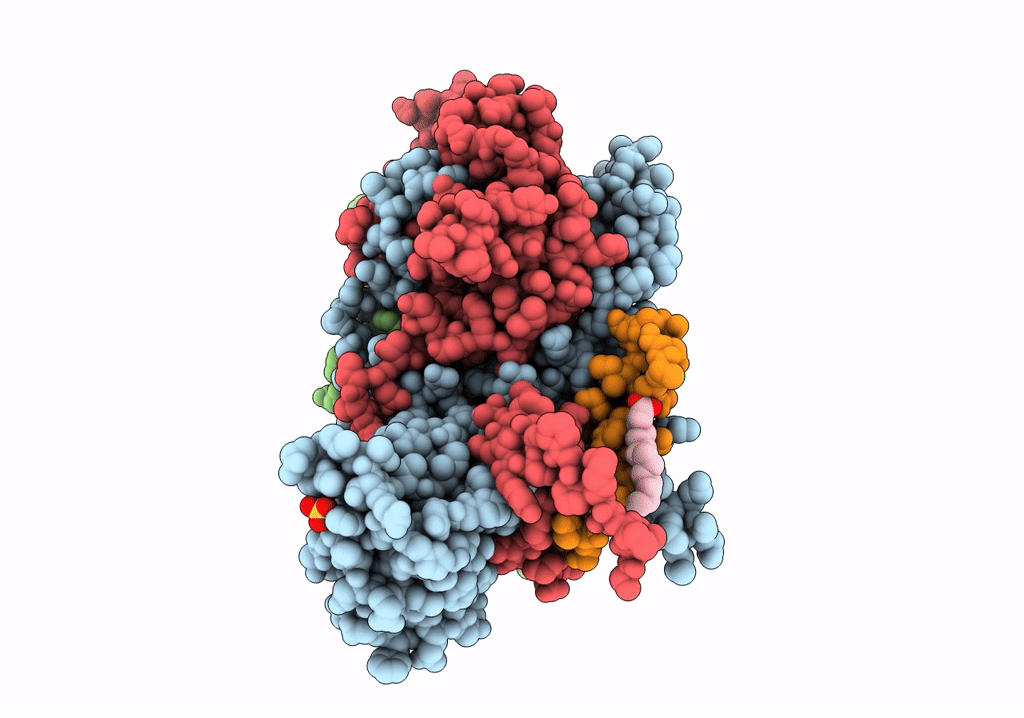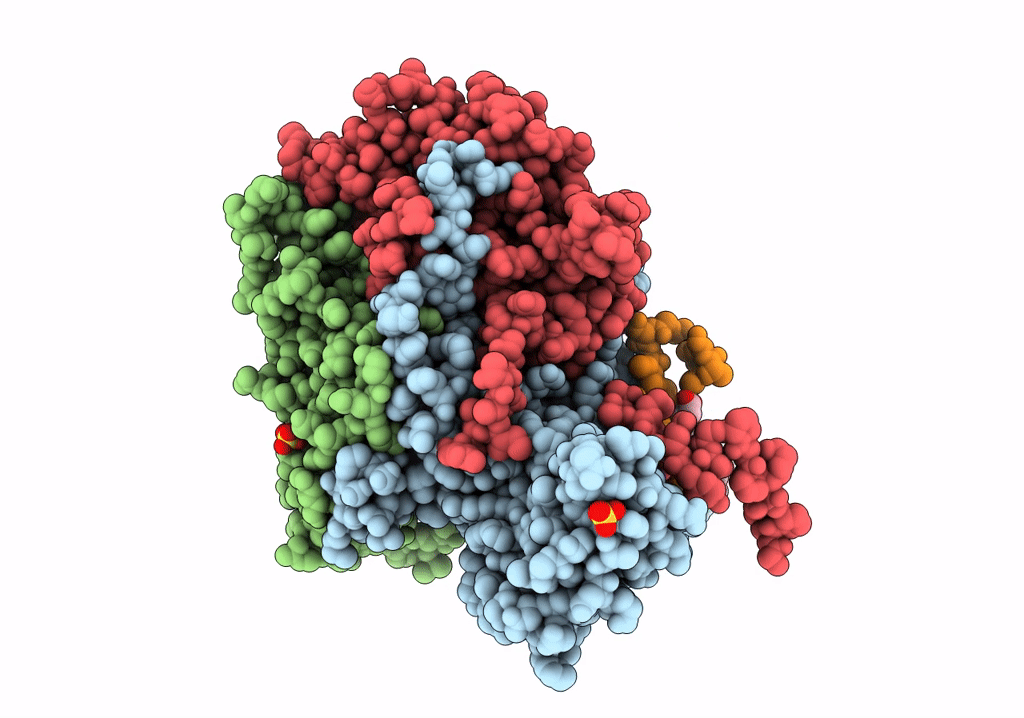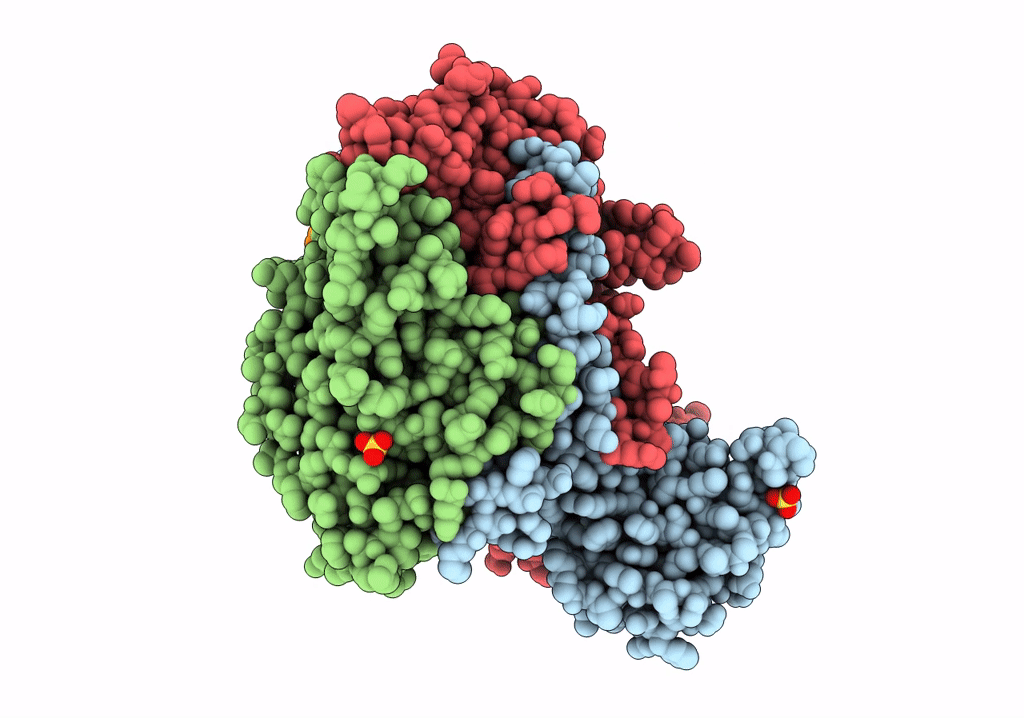
PDB ID:
6THN
Keywords:
Title:
Multiple Genomic RNA-Coat Protein Contacts Play Vital Roles in the Assembly of Infectious Enterovirus-E symmetry expansion+2fold focused classification
Biological Source:
Source Organism(s):
Bovine enterovirus (strain VG-5-27) (Taxon ID: 12065)
Method Details:
Experimental Method:
Resolution:
2.60 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE