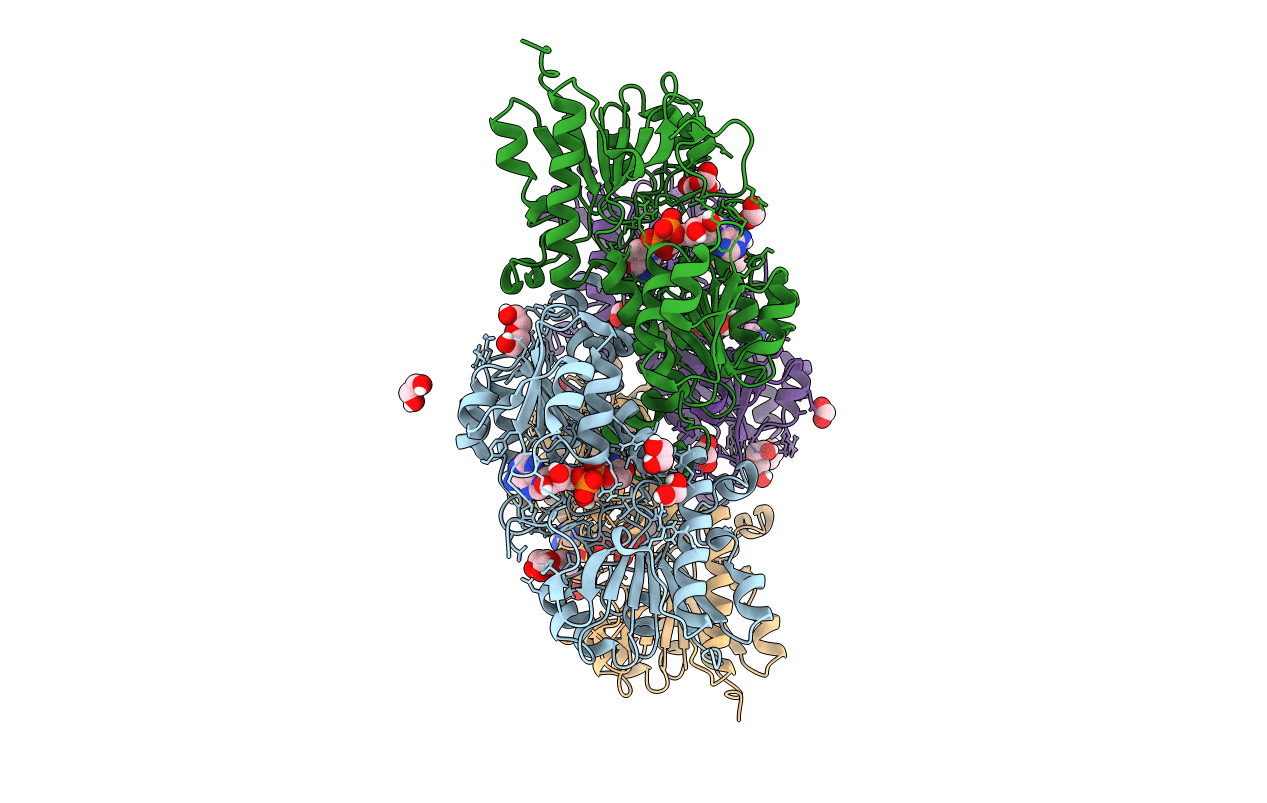
Deposition Date
2019-10-25
Release Date
2020-11-18
Last Version Date
2024-01-24
Entry Detail
PDB ID:
6T92
Keywords:
Title:
NAD+-dependent fungal formate dehydrogenase from Chaetomium thermophilum: A complex of N120C mutant protein with the reduced form of the cofactor NADH and the substrate formate at a secondary site.
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
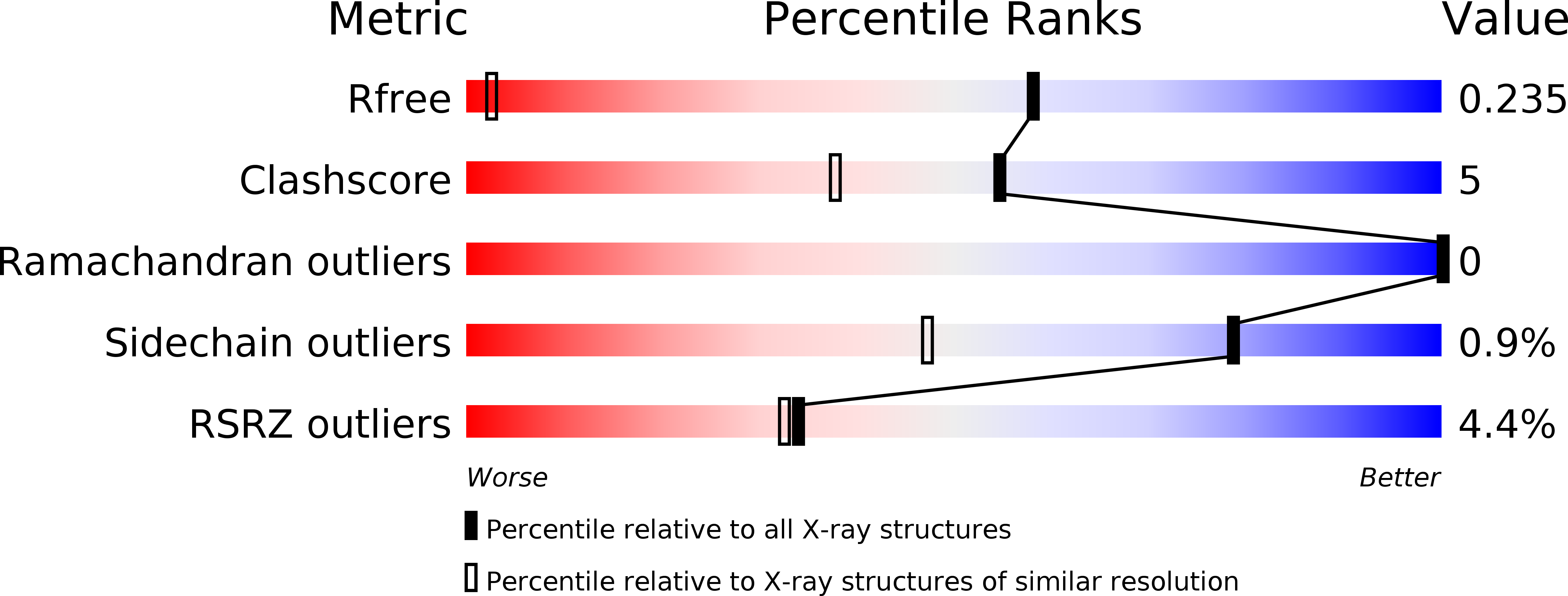
Resolution:
1.12 Å
R-Value Free:
0.22
R-Value Work:
0.20
Space Group:
P 1