Deposition Date
2019-10-11
Release Date
2020-05-13
Last Version Date
2024-01-24
Entry Detail
PDB ID:
6T3S
Keywords:
Title:
Structure of Oceanobacillus iheyensis group II intron U-mutant (C289U/C358U/G385A) in the presence of Na+, Mg2+ and 5'-exon
Biological Source:
Source Organism(s):
Oceanobacillus iheyensis (Taxon ID: 182710)
Method Details:
Experimental Method:
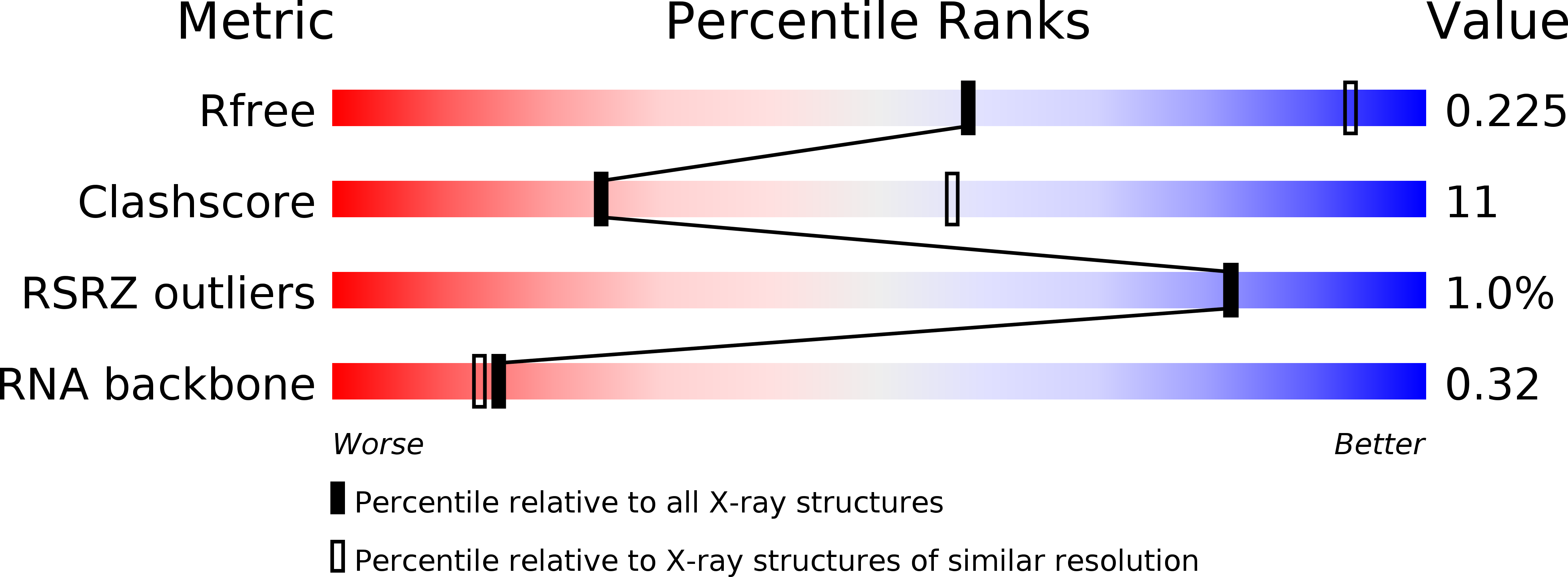
Resolution:
3.28 Å
R-Value Free:
0.22
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 21 21 21