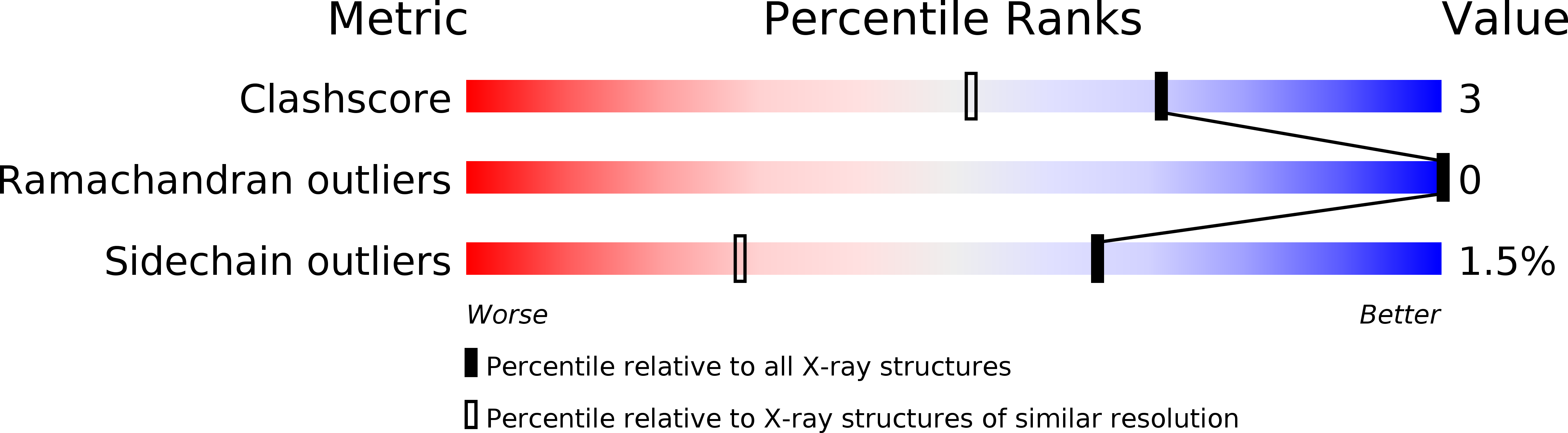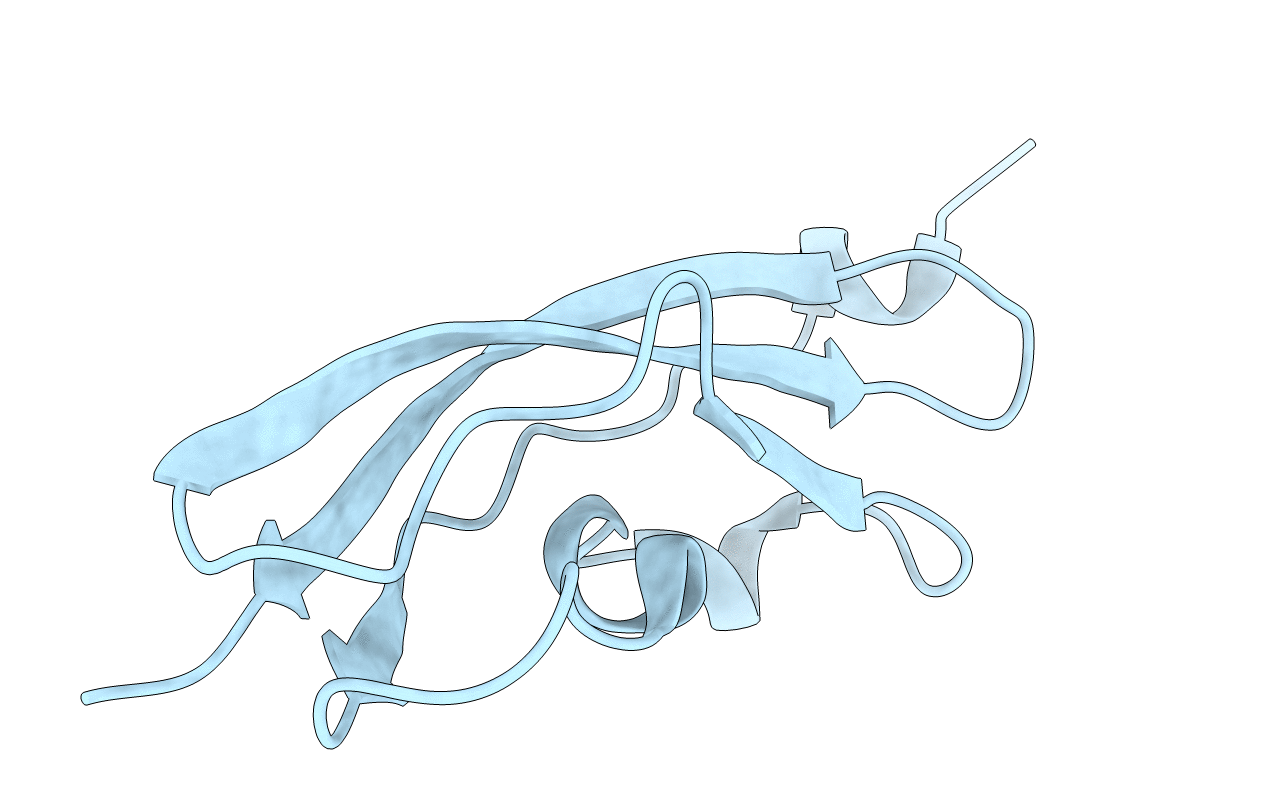
Deposition Date
2019-09-24
Release Date
2019-12-18
Last Version Date
2024-06-19
Entry Detail
PDB ID:
6SX1
Keywords:
Title:
Structure of Rib Standard, a Rib domain from Lactobacillus acidophilus
Biological Source:
Source Organism(s):
Expression System(s):
Method Details: