Deposition Date
2019-09-23
Release Date
2020-04-15
Last Version Date
2024-11-20
Entry Detail
PDB ID:
6SWR
Keywords:
Title:
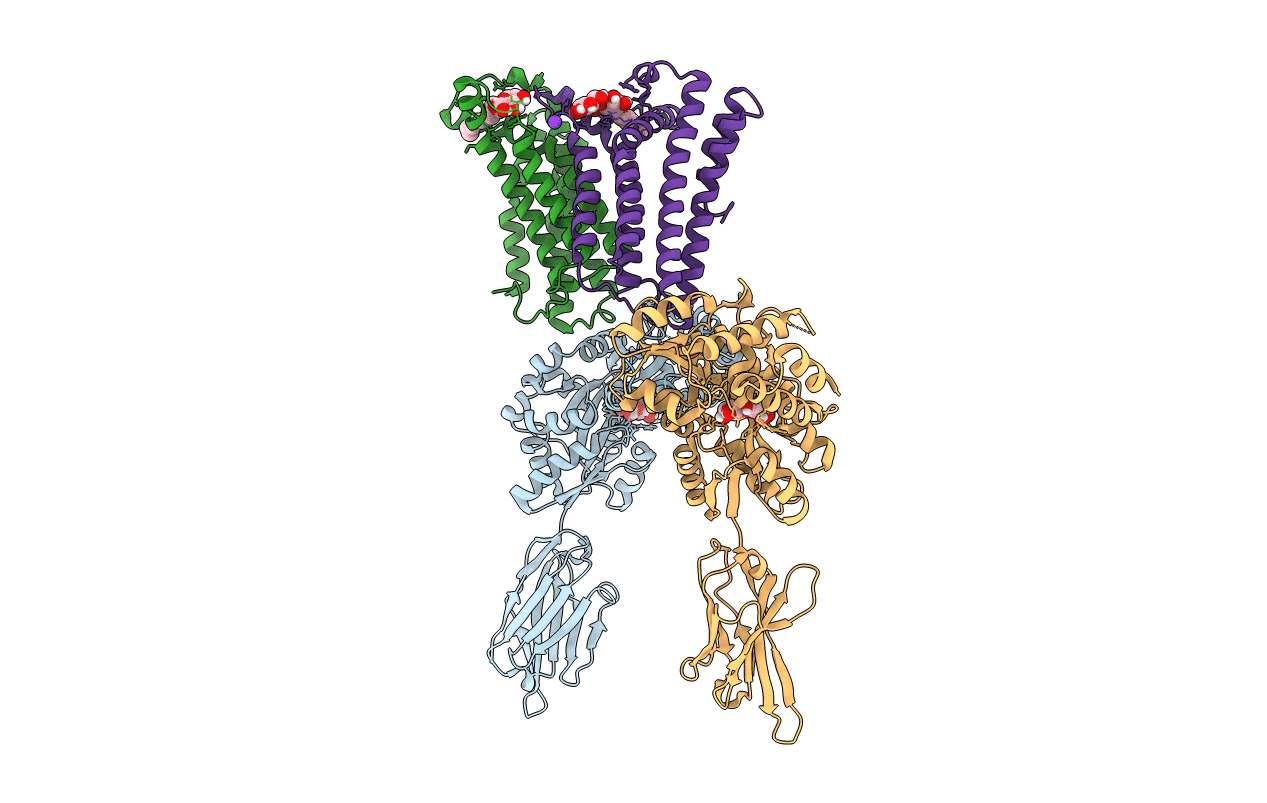
Crystal structure of the lysosomal potassium channel MtTMEM175 T38A mutant soaked with zinc
Biological Source:
Source Organism(s):
Lama glama (Taxon ID: 9844)
Escherichia coli (strain K12) (Taxon ID: 83333)
Marivirga tractuosa (strain ATCC 23168 / DSM 4126 / NBRC 15989 / NCIMB 1408 / VKM B-1430 / H-43) (Taxon ID: 643867)
Escherichia coli (strain K12) (Taxon ID: 83333)
Marivirga tractuosa (strain ATCC 23168 / DSM 4126 / NBRC 15989 / NCIMB 1408 / VKM B-1430 / H-43) (Taxon ID: 643867)
Expression System(s):
Method Details:
Experimental Method:
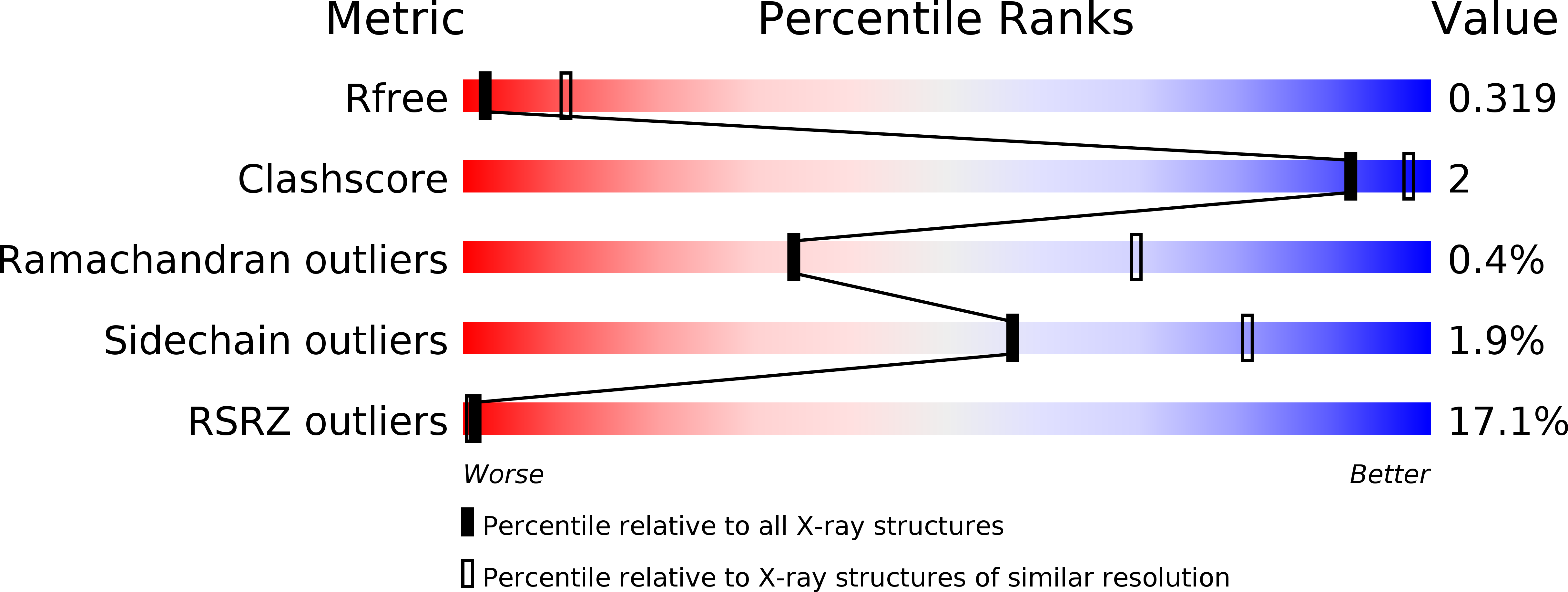
Resolution:
3.20 Å
R-Value Free:
0.29
R-Value Work:
0.26
Space Group:
P 21 2 21