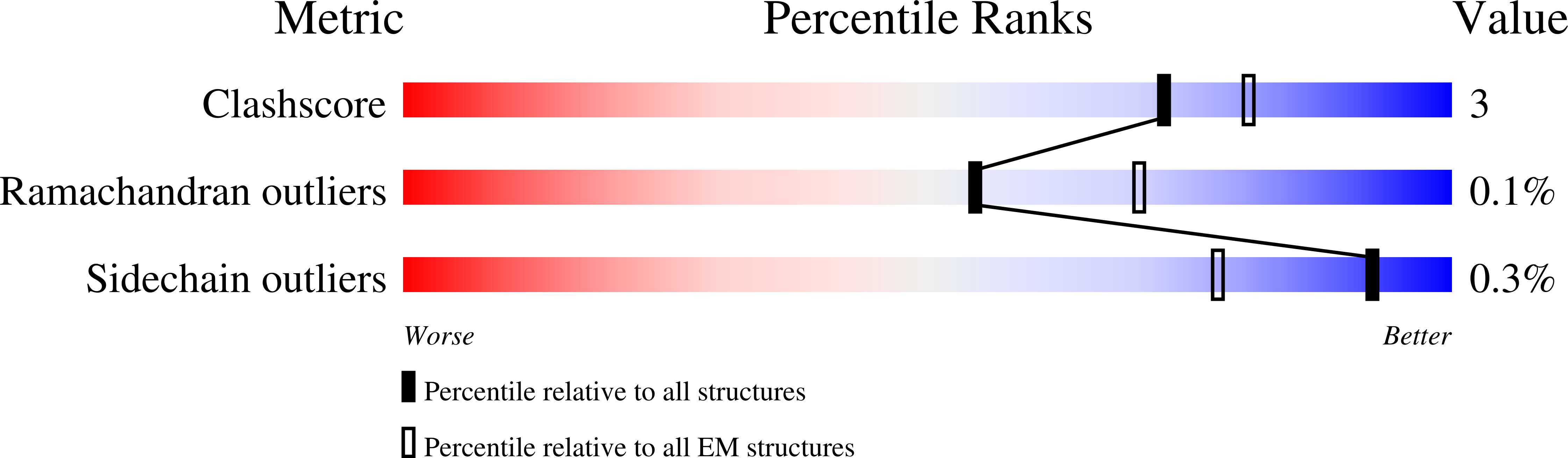
Deposition Date
2019-08-22
Release Date
2020-05-20
Last Version Date
2025-10-01
Entry Detail
PDB ID:
6SML
Keywords:
Title:
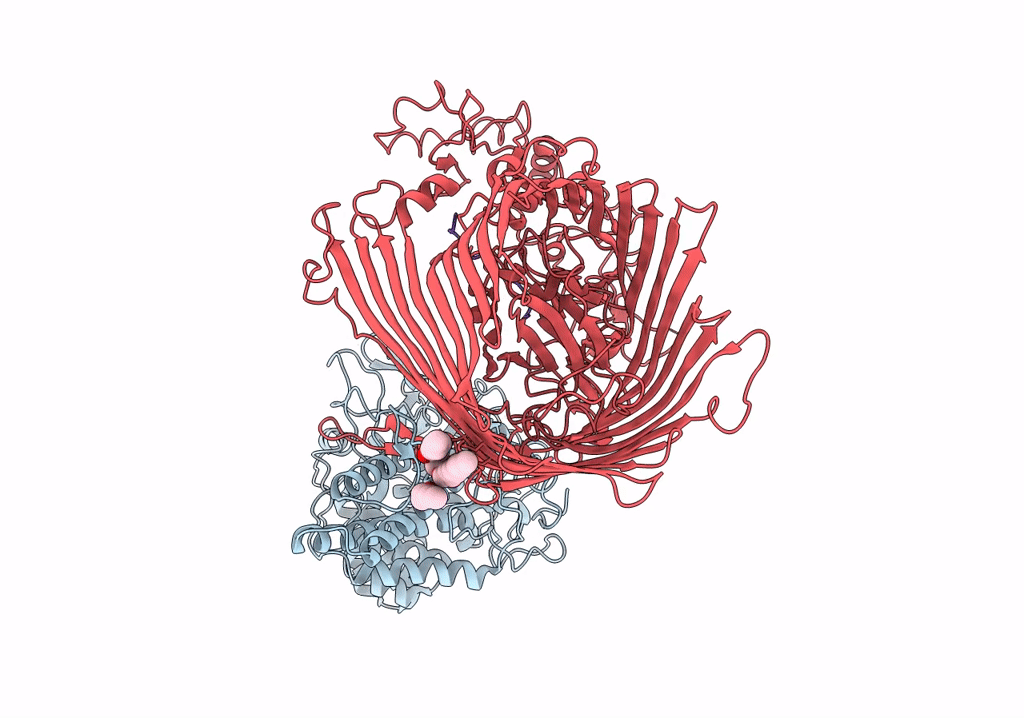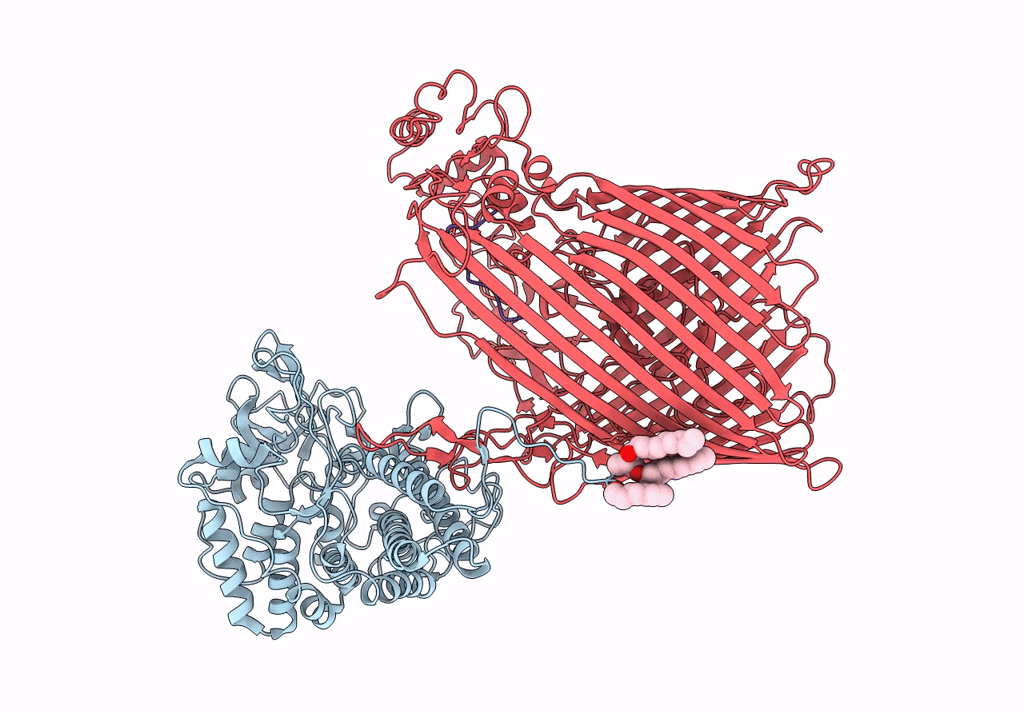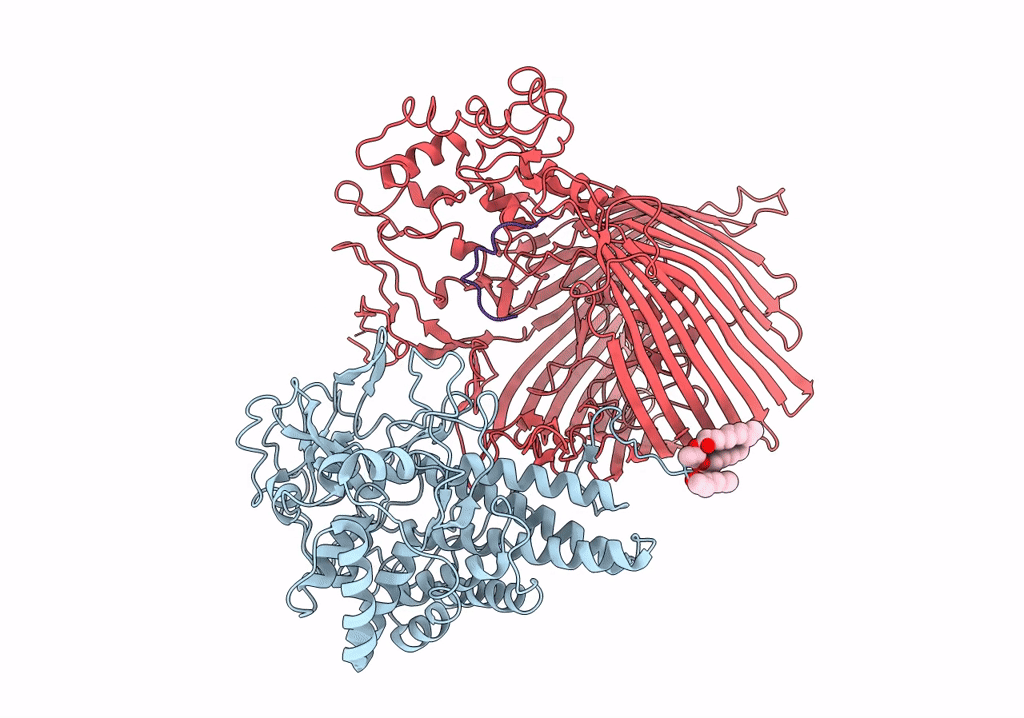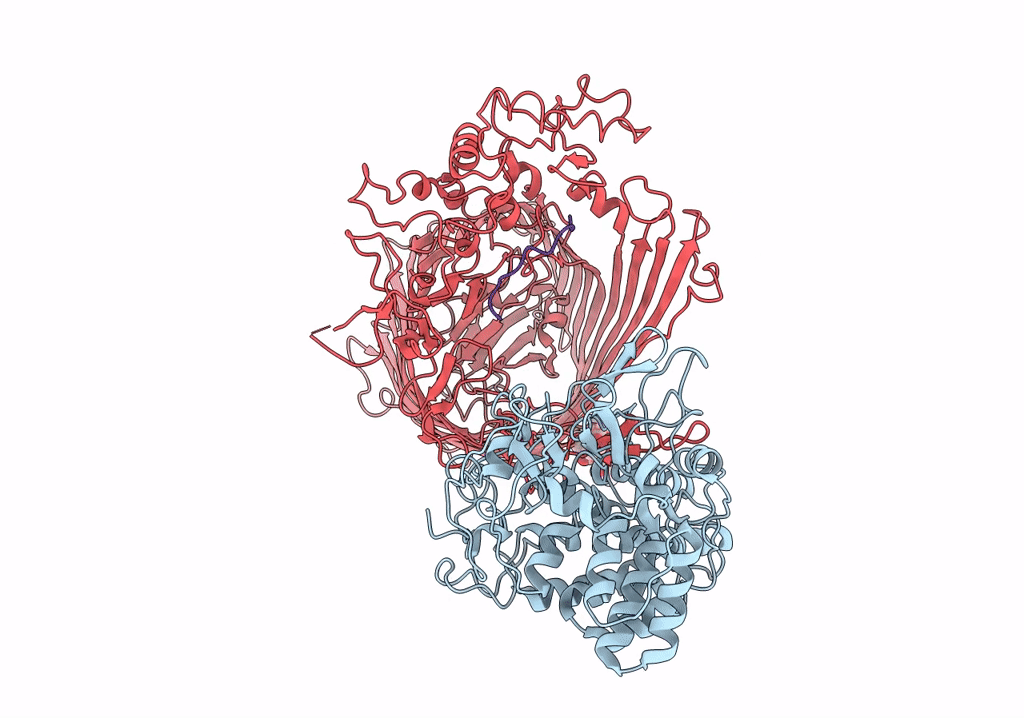
Structure of the RagAB peptide importer in the 'open-open' state
Biological Source:
Source Organism(s):
Porphyromonas gingivalis W83 (Taxon ID: 242619)
Method Details:
Experimental Method:
Resolution:
3.40 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE