Deposition Date
2019-07-18
Release Date
2019-12-11
Last Version Date
2024-10-16
Entry Detail
PDB ID:
6SAW
Keywords:
Title:
Chromophore binding domain of bacteriophytochrome linked diguanylyl cyclase from Idiomarina species A28L (dimeric Pfr-like state).
Biological Source:
Source Organism(s):
Idiomarina sp. A28L (Taxon ID: 1036674)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.00 Å
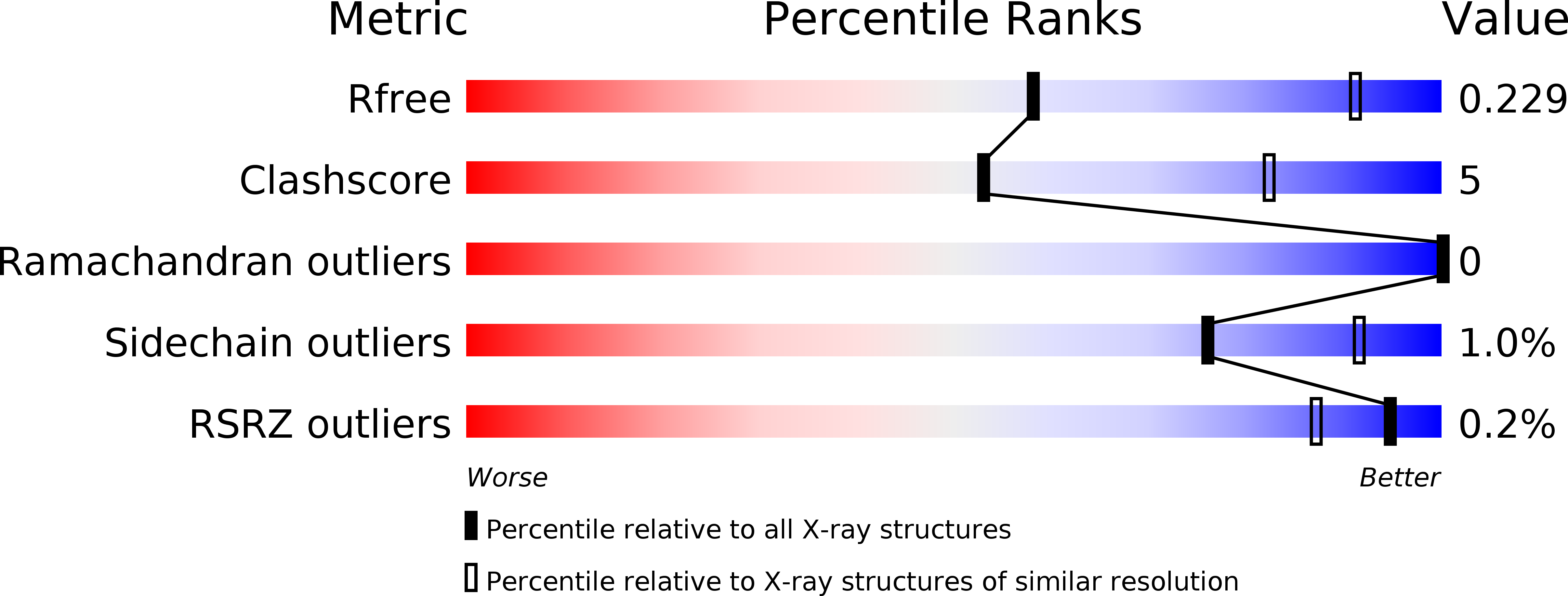
R-Value Free:
0.22
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
H 3