Deposition Date
2019-07-08
Release Date
2019-09-04
Last Version Date
2024-05-22
Entry Detail
PDB ID:
6S85
Keywords:
Title:
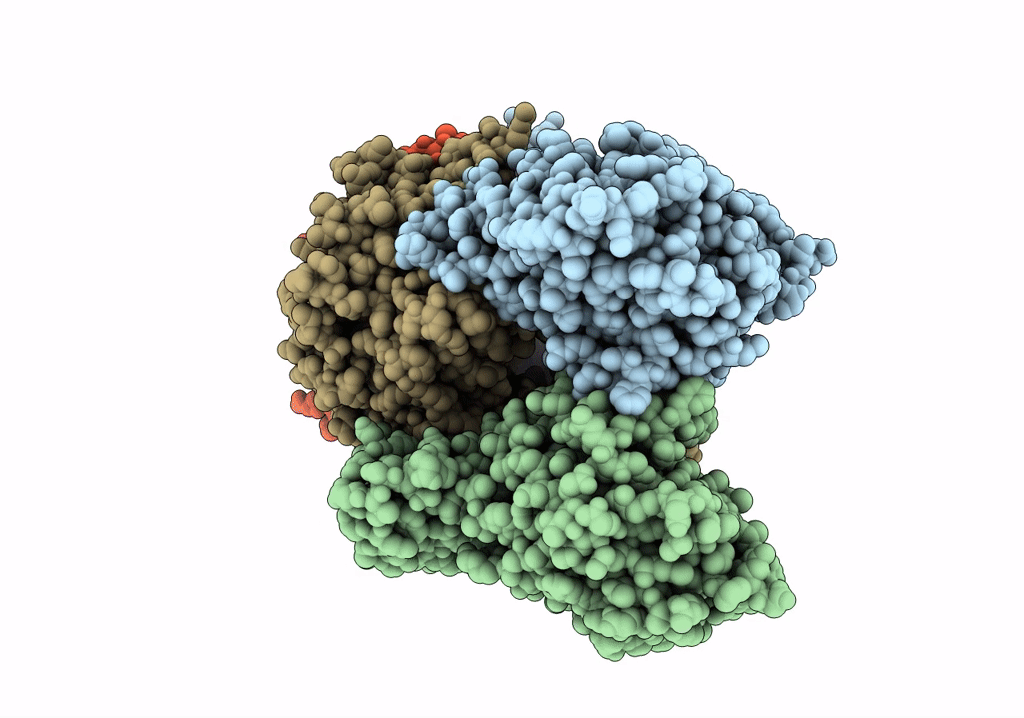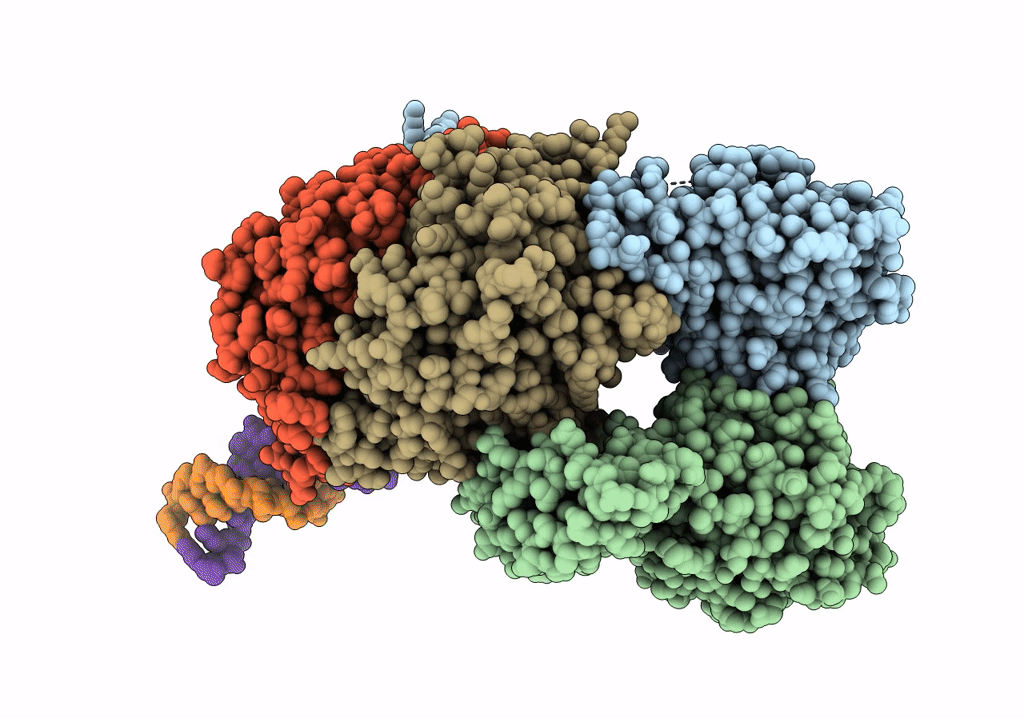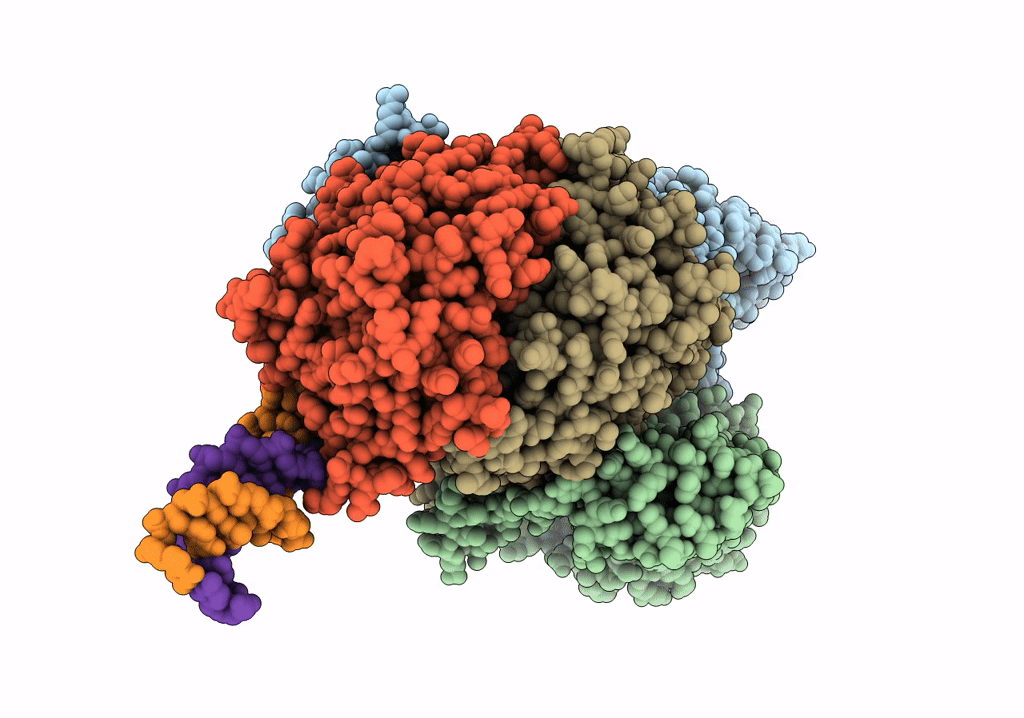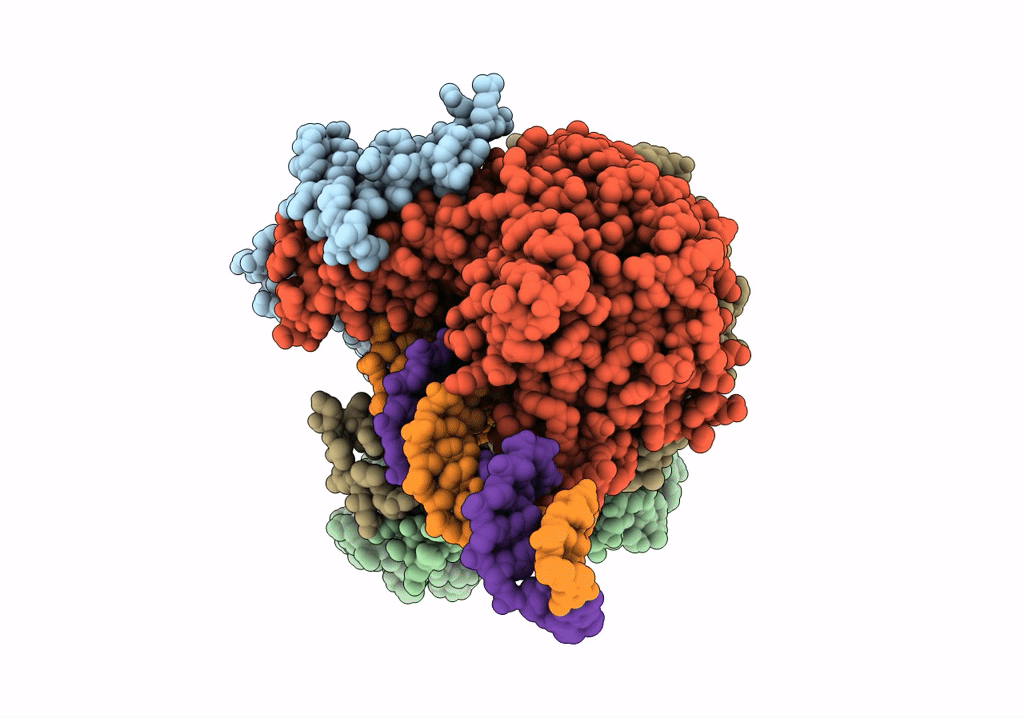
Cutting state of the E. coli Mre11-Rad50 (SbcCD) head complex bound to ADP and dsDNA.
Biological Source:
Source Organism(s):
Escherichia coli (Taxon ID: 562)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
4.20 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


