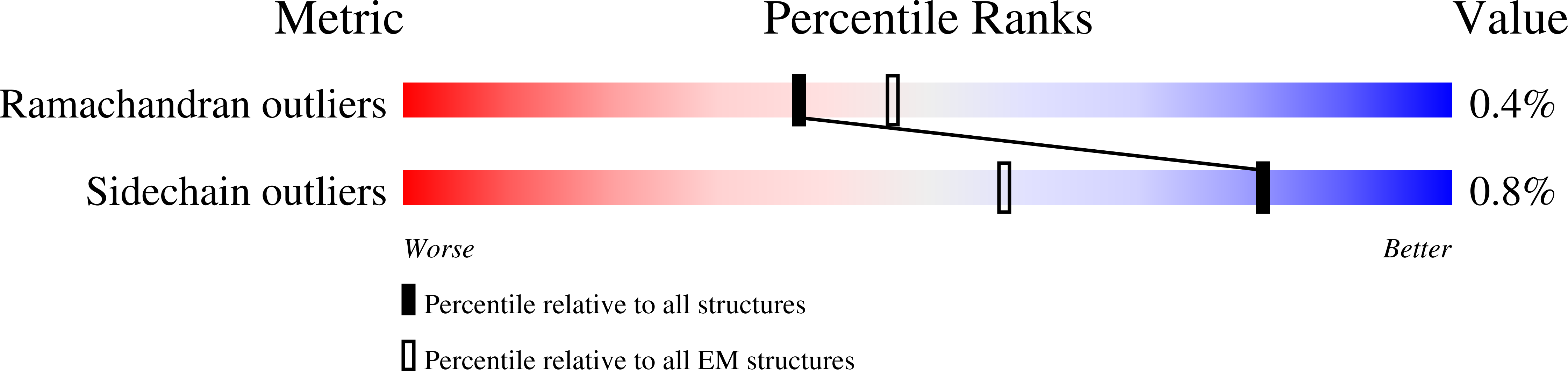Deposition Date
2019-06-18
Release Date
2020-01-22
Last Version Date
2024-05-15
Entry Detail
PDB ID:
6S1K
Keywords:
Title:
E. coli Core Signaling Unit, carrying QQQQ receptor mutation
Biological Source:
Source Organism(s):
Escherichia coli str. K-12 substr. MG1655star (Taxon ID: 879462)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
8.38 Å
Aggregation State:
2D ARRAY
Reconstruction Method:
SUBTOMOGRAM AVERAGING