Deposition Date
2019-06-08
Release Date
2020-04-15
Last Version Date
2024-10-09
Entry Detail
PDB ID:
6RXQ
Keywords:
Title:
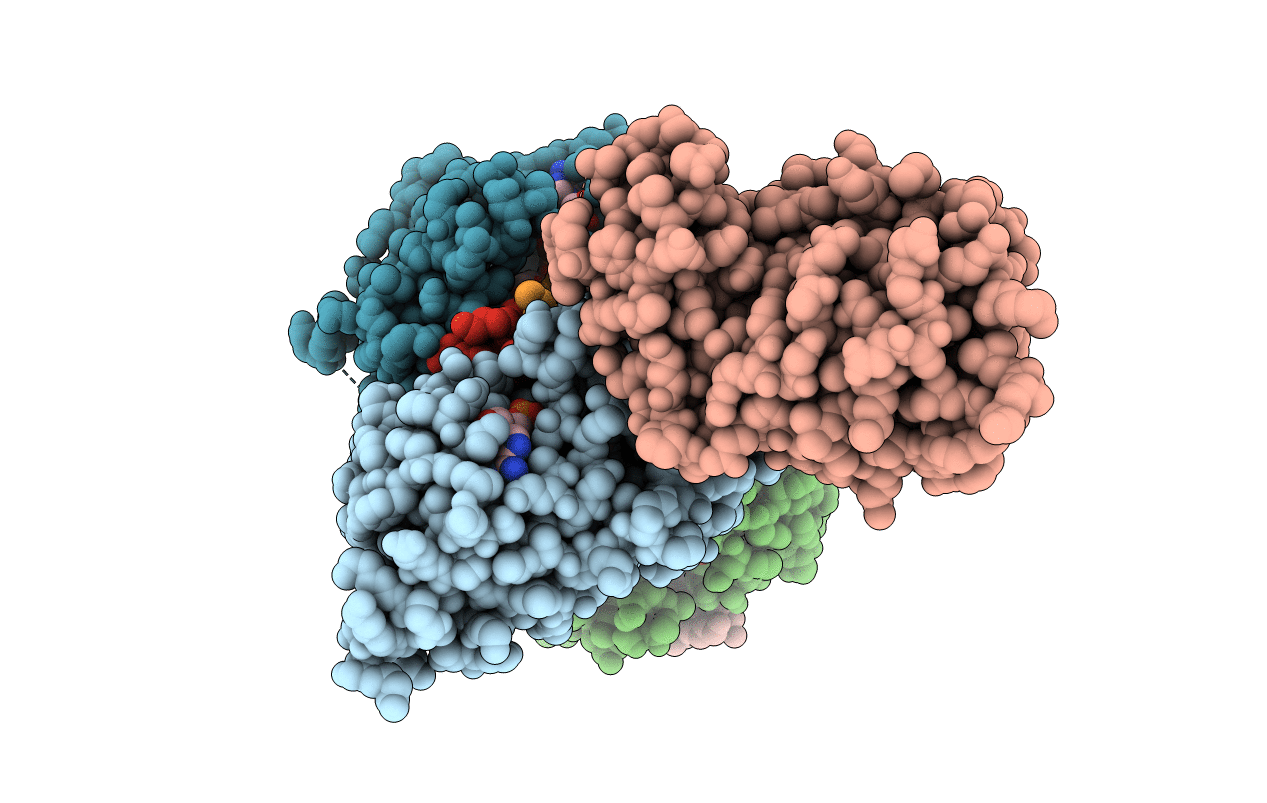
Crystal structure of CobB Ac2 (A76G,I131C,V162A) in complex with H4K16Cr-2'OH-ADPr peptide intermediate after soaking
Biological Source:
Source Organism(s):
Escherichia coli (strain K12) (Taxon ID: 83333)
Saccharomyces cerevisiae (strain ATCC 204508 / S288c) (Taxon ID: 559292)
Saccharomyces cerevisiae (strain ATCC 204508 / S288c) (Taxon ID: 559292)
Expression System(s):
Method Details:
Experimental Method:
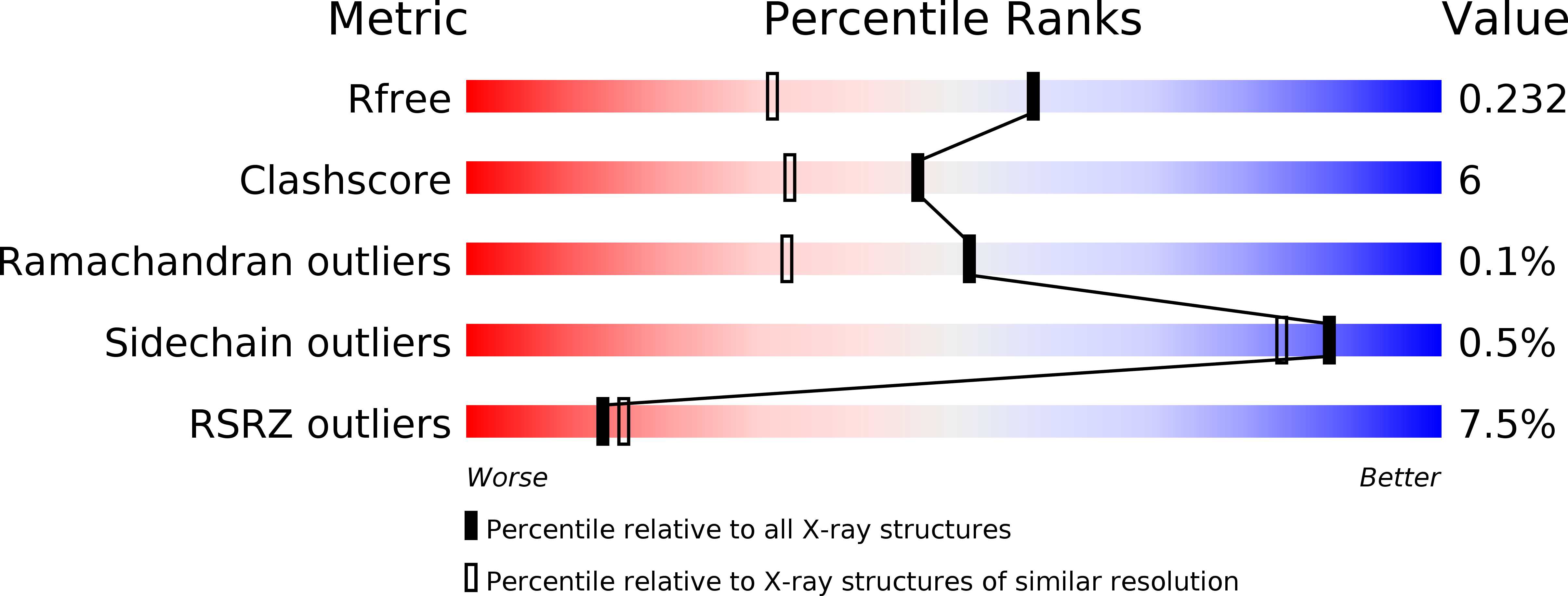
Resolution:
1.70 Å
R-Value Free:
0.22
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 1 21 1