Deposition Date
2019-05-28
Release Date
2020-06-24
Last Version Date
2024-11-13
Entry Detail
PDB ID:
6RUQ
Keywords:
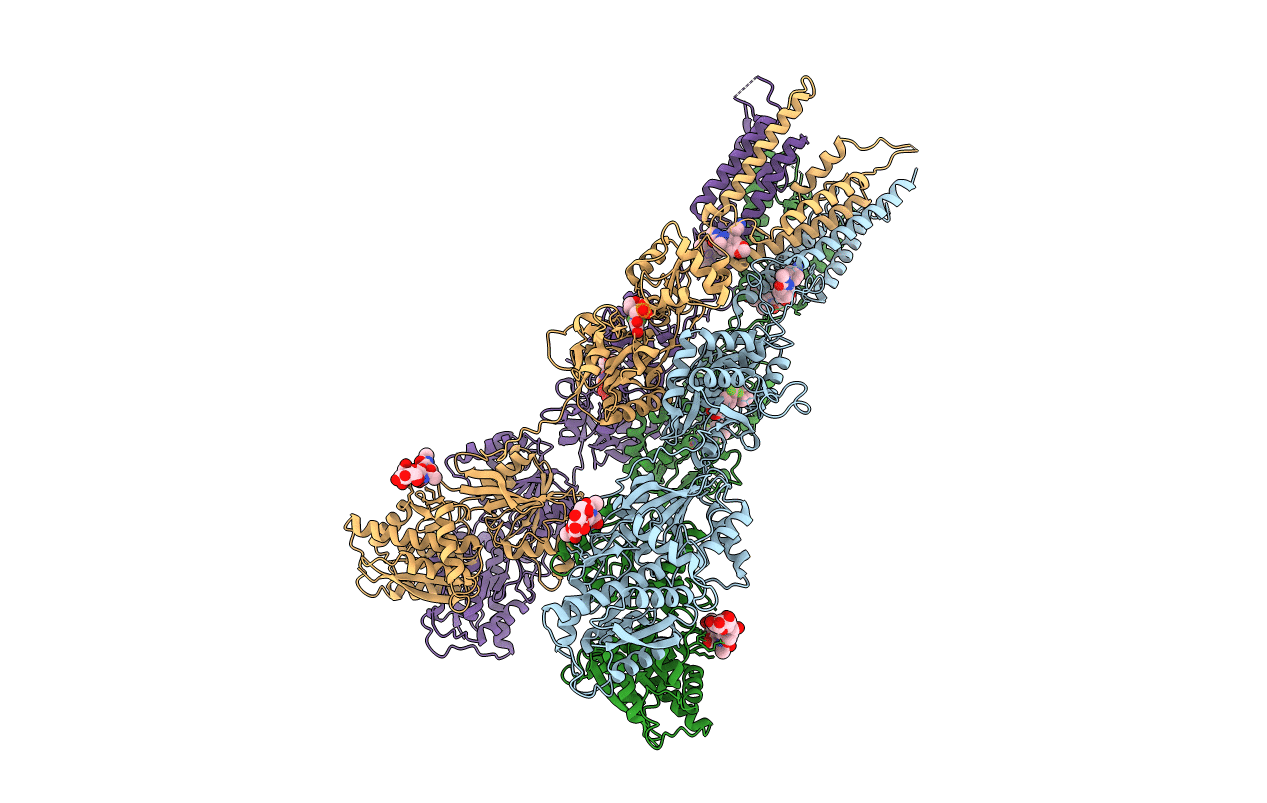
Title:
Structure of GluA2cryst in complex the antagonist ZK200775 and the negative allosteric modulator GYKI53655 at 4.65 A resolution
Biological Source:
Source Organism:
Rattus norvegicus (Taxon ID: 10116)
Host Organism:
Method Details:
Experimental Method:
Resolution:
4.65 Å
R-Value Free:
0.29
R-Value Work:
0.24
R-Value Observed:
0.24
Space Group:
P 1 21 1


