Deposition Date
2019-05-16
Release Date
2019-08-21
Last Version Date
2024-01-24
Entry Detail
PDB ID:
6RR3
Keywords:
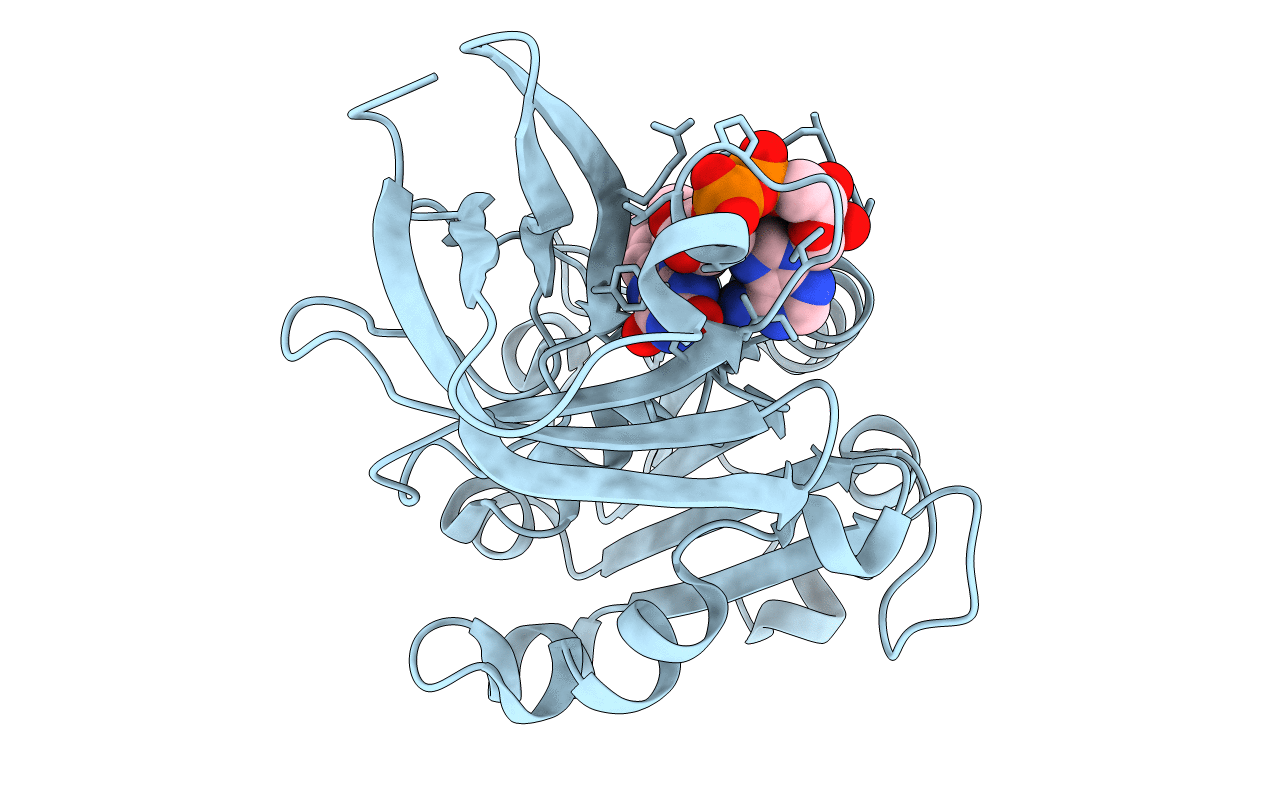
Title:
CRYSTAL STRUCTURE OF FAD-CONTAINING FERREDOXIN-NADP REDUCTASE FROM BRUCELLA OVIS
Biological Source:
Source Organism(s):
Brucella ovis ATCC 25840 (Taxon ID: 444178)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
1.69 Å
R-Value Free:
0.21
R-Value Work:
0.16
R-Value Observed:
0.17
Space Group:
P 41


