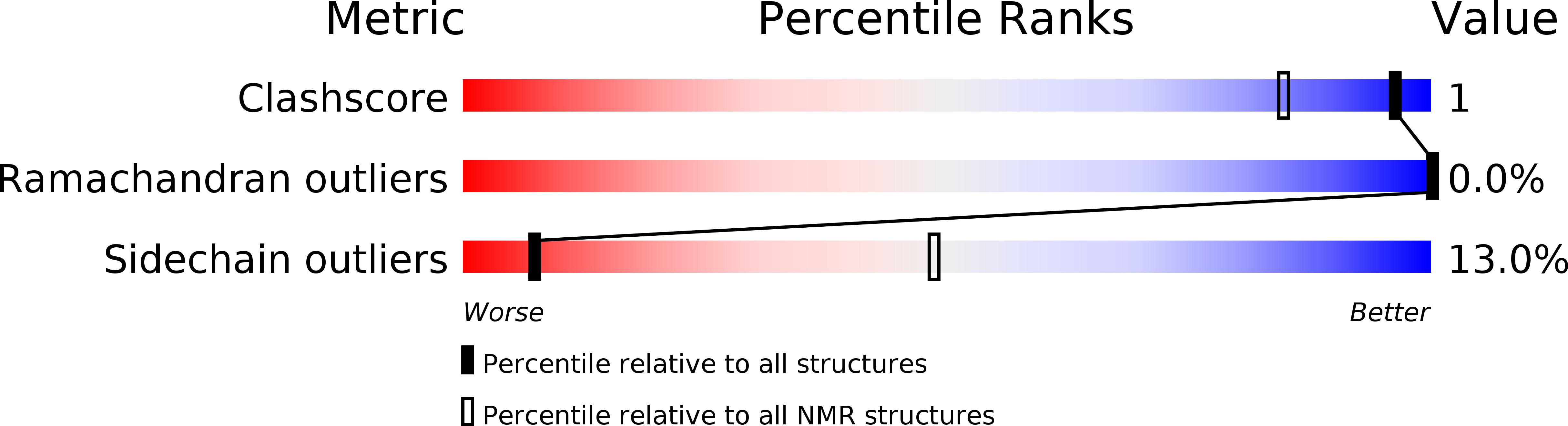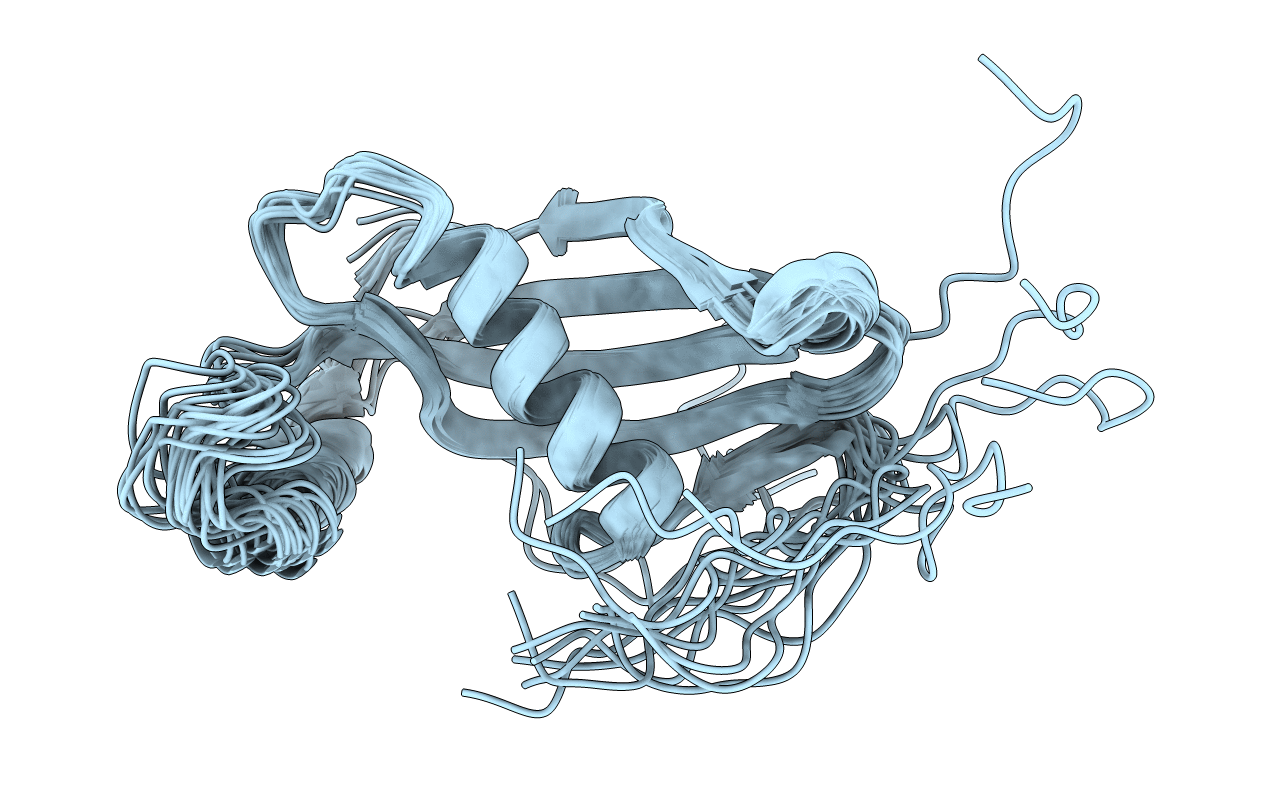
Deposition Date
2019-05-14
Release Date
2019-07-31
Last Version Date
2024-11-13
Entry Detail
PDB ID:
6RPV
Keywords:
Title:
Extremely stable monomeric variant of human cystatin C with single amino acid substitution
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
20
Conformers Submitted:
20
Selection Criteria:
target function