Deposition Date
2019-05-09
Release Date
2020-04-15
Last Version Date
2024-10-09
Entry Detail
PDB ID:
6RNR
Keywords:
Title:
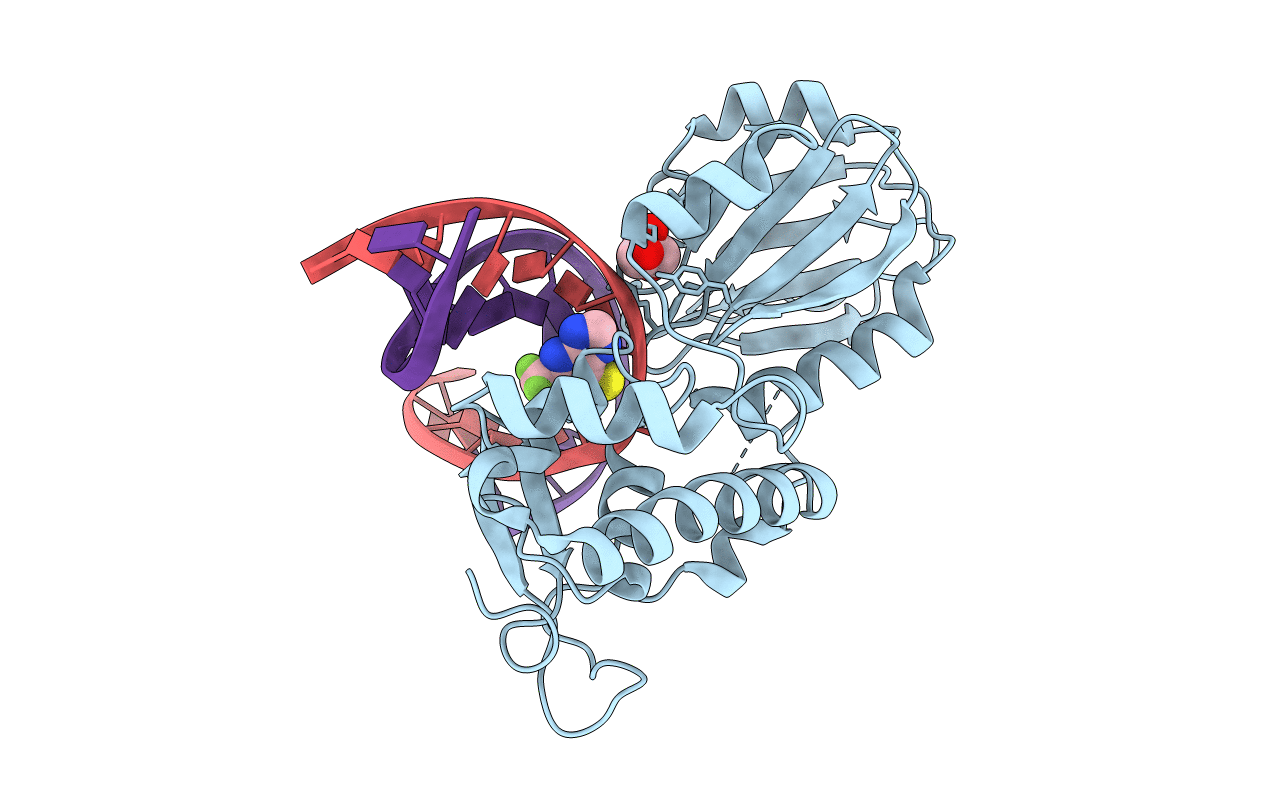
The crystal structure of a complex between the LlFpg protein, a THF-DNA and an inhibitor
Biological Source:
Source Organism:
Lactococcus lactis subsp. cremoris (Taxon ID: 1359)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Host Organism:
Method Details:
Experimental Method:
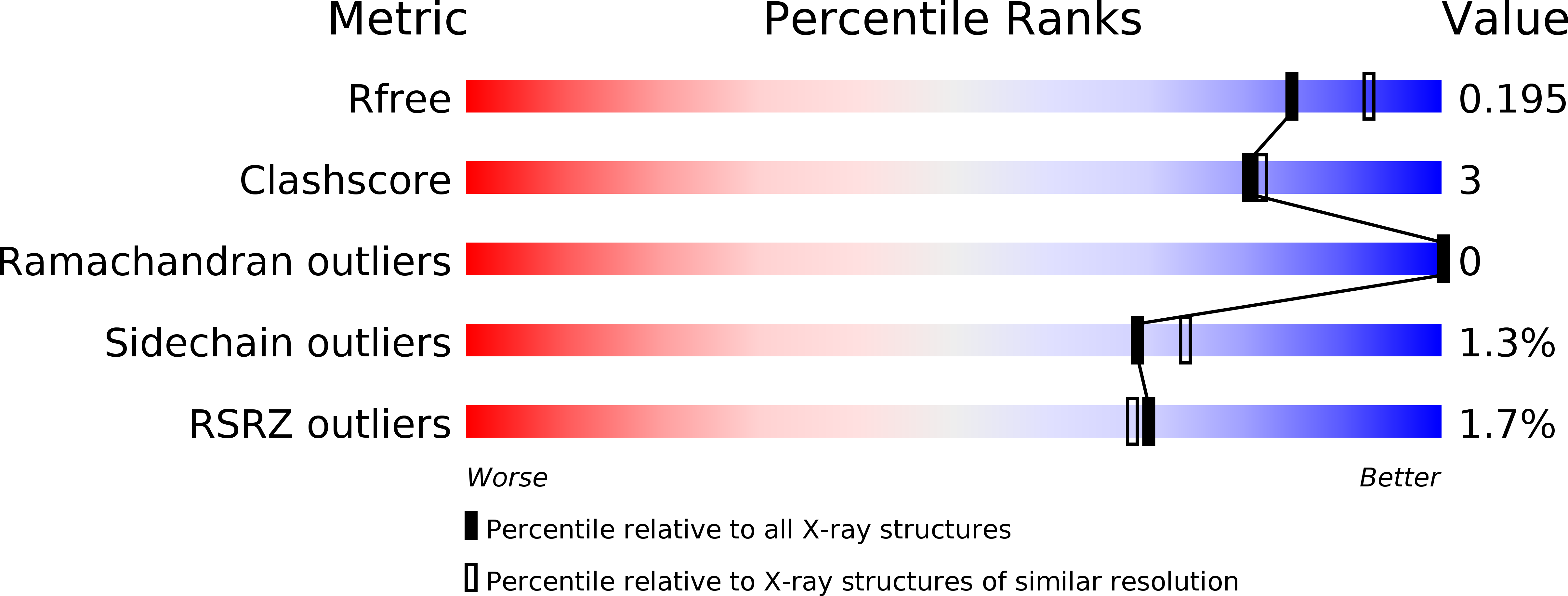
Resolution:
2.00 Å
R-Value Free:
0.19
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 41 21 2