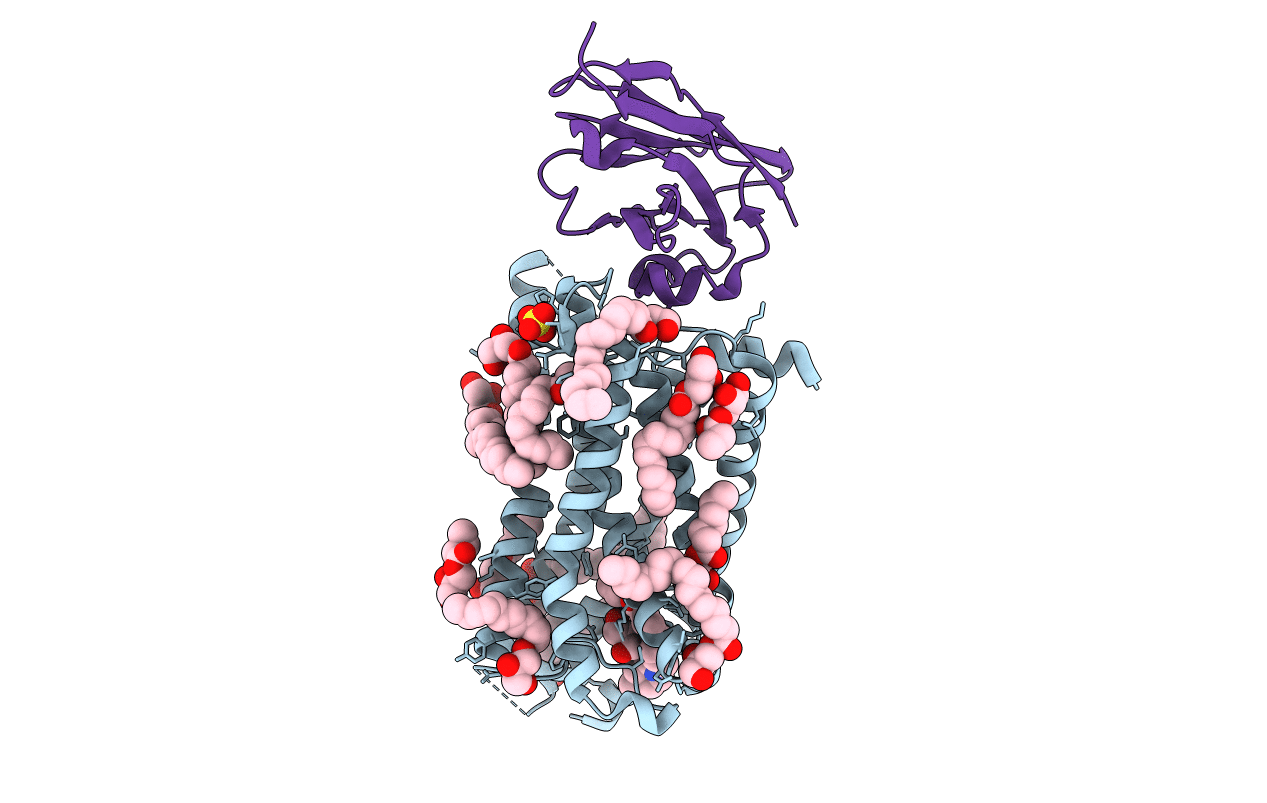
Deposition Date
2019-05-08
Release Date
2019-08-14
Last Version Date
2024-11-20
Entry Detail
PDB ID:
6RNK
Keywords:
Title:
Crystal structure of a humanized (K18E, K269N) rat succinate receptor SUCNR1 (GPR91) in complex with a nanobody and antagonist NF-56-EJ40.
Biological Source:
Source Organism(s):
Rattus norvegicus (Taxon ID: 10116)
Vicugna pacos (Taxon ID: 30538)
Vicugna pacos (Taxon ID: 30538)
Expression System(s):
Method Details:
Experimental Method:
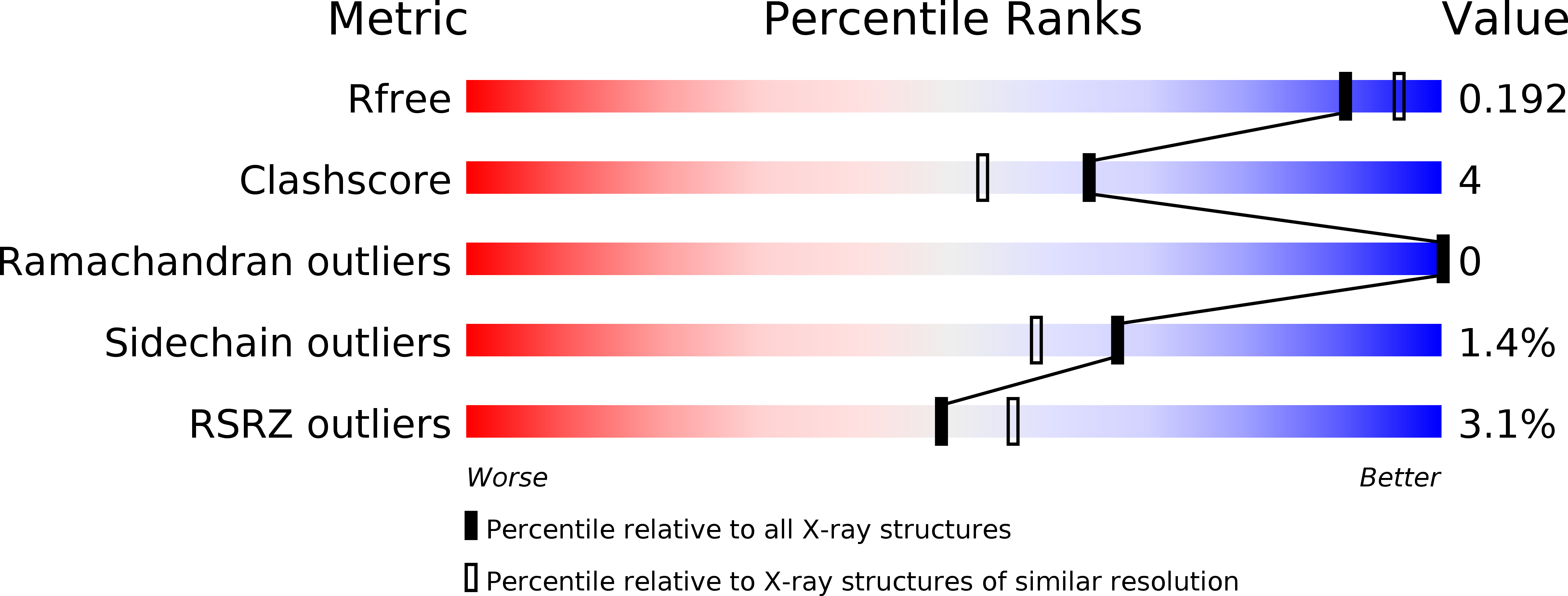
Resolution:
1.94 Å
R-Value Free:
0.19
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
C 1 2 1