Deposition Date
2019-03-28
Release Date
2019-07-03
Last Version Date
2024-01-24
Entry Detail
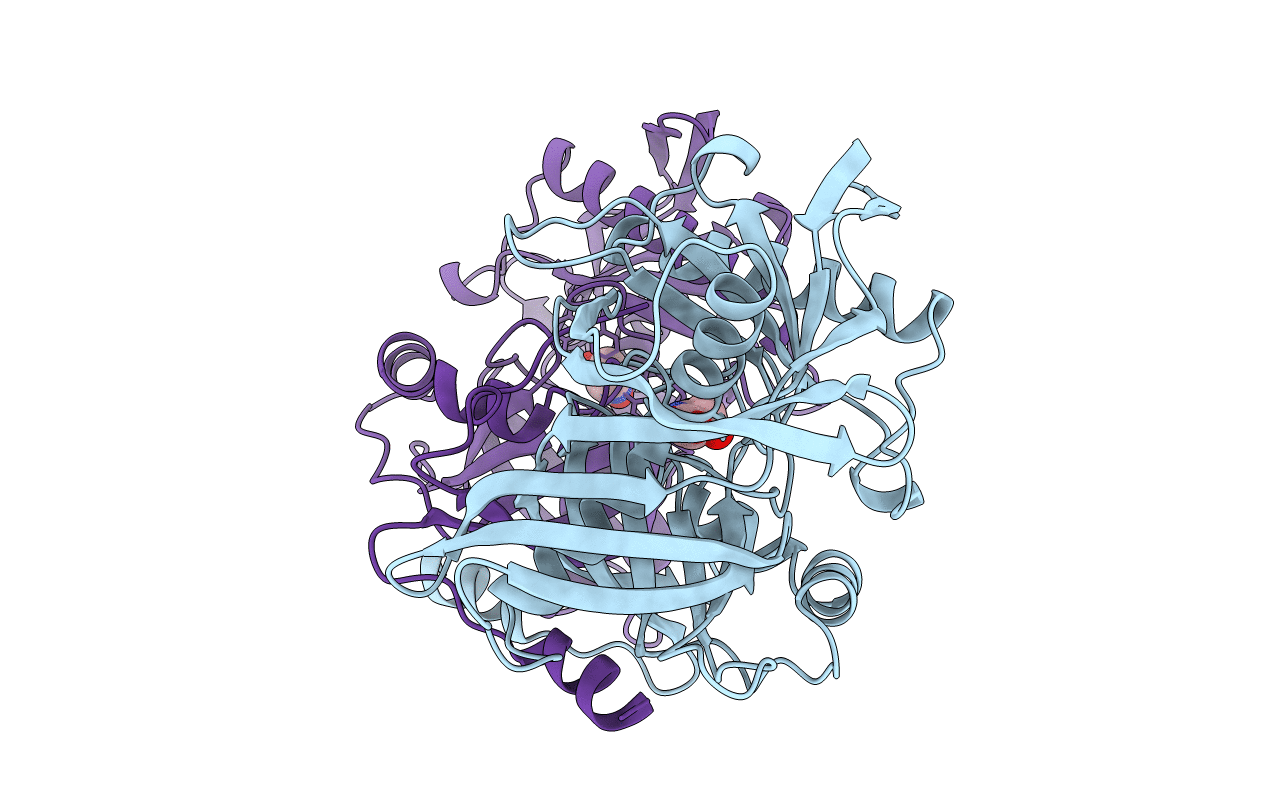
PDB ID:
6R77
Keywords:
Title:
Crystal structure of trans-3-Hydroxy-L-proline dehydratase in complex with substrate - closed conformation
Biological Source:
Source Organism(s):
Thermococcus litoralis (Taxon ID: 2265)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.00 Å
R-Value Free:
0.21
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 21 21 21


