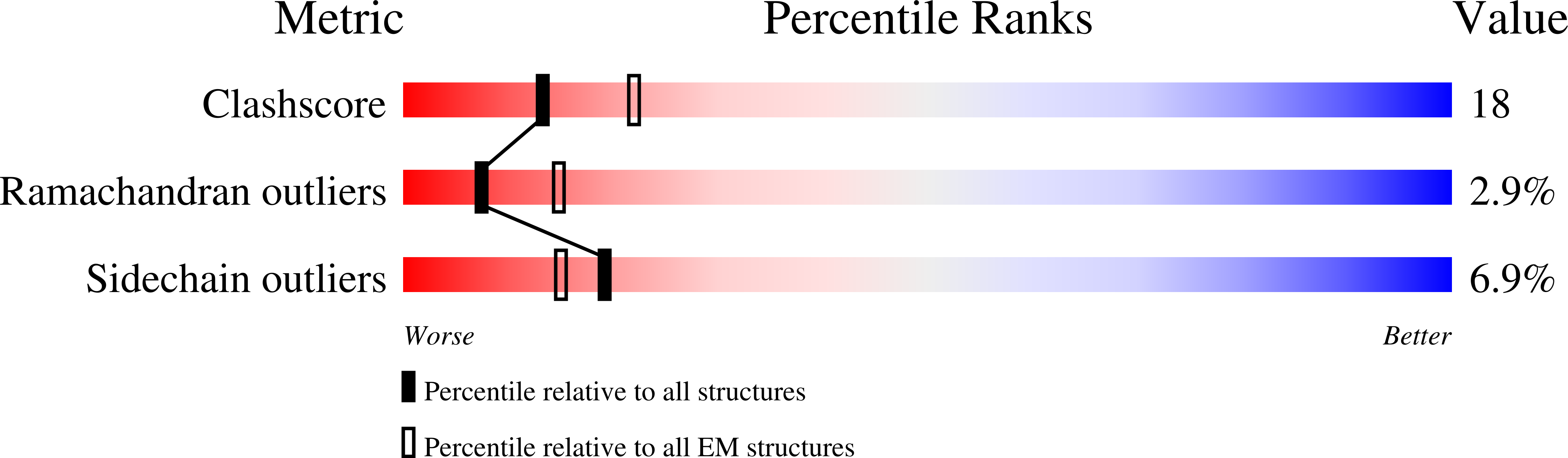
Deposition Date
2019-03-15
Release Date
2019-04-24
Last Version Date
2024-05-15
Entry Detail
PDB ID:
6R1T
Keywords:
Title:
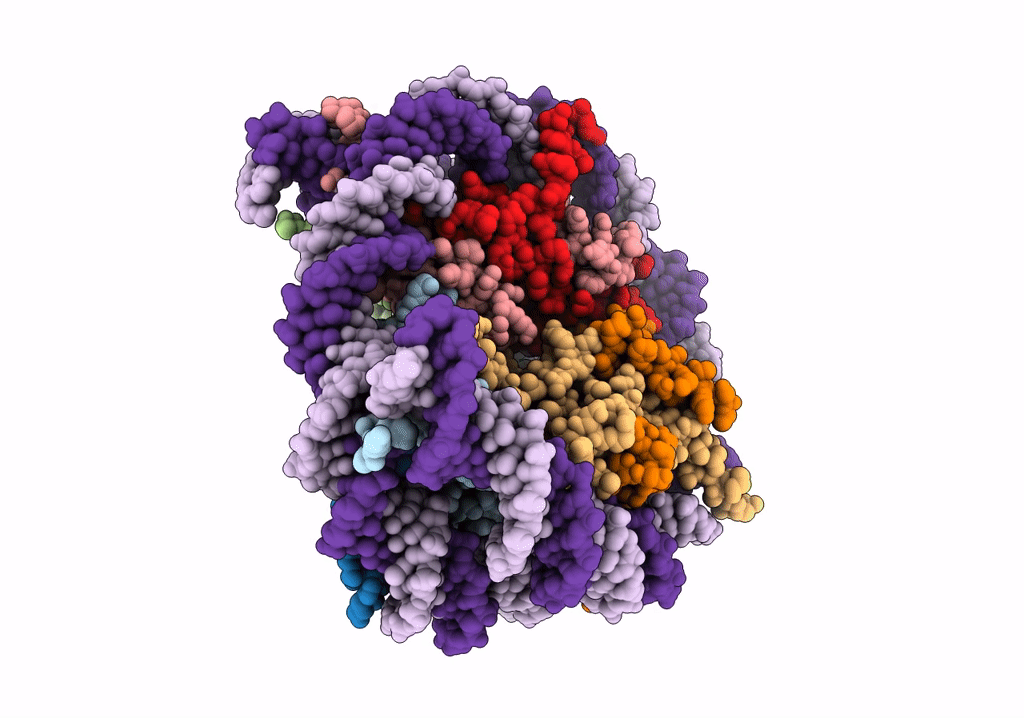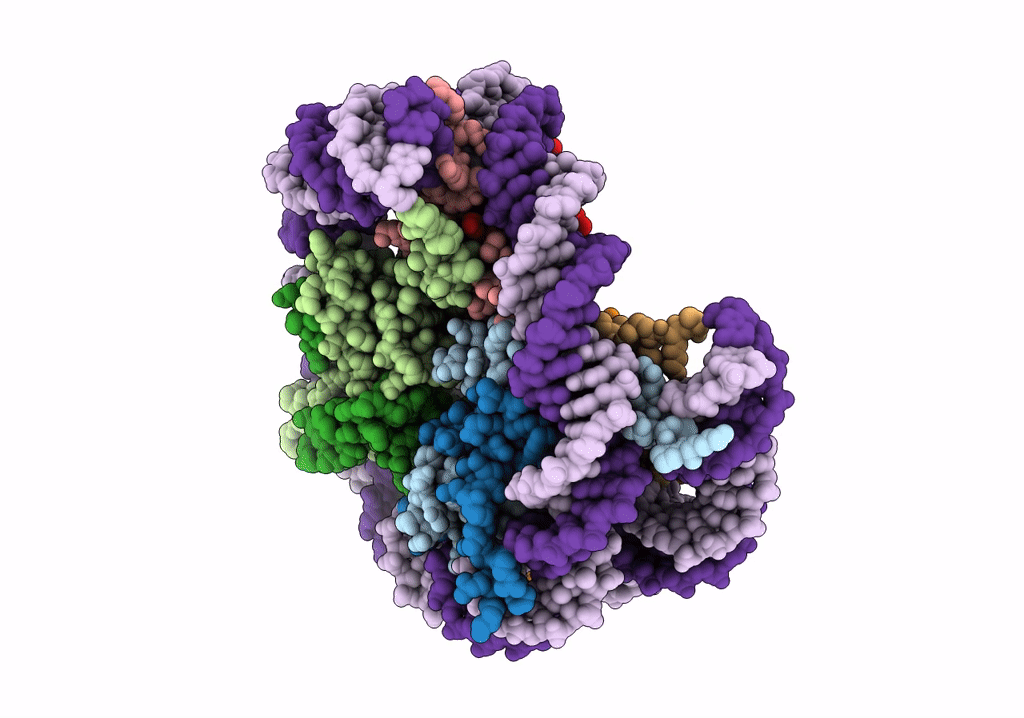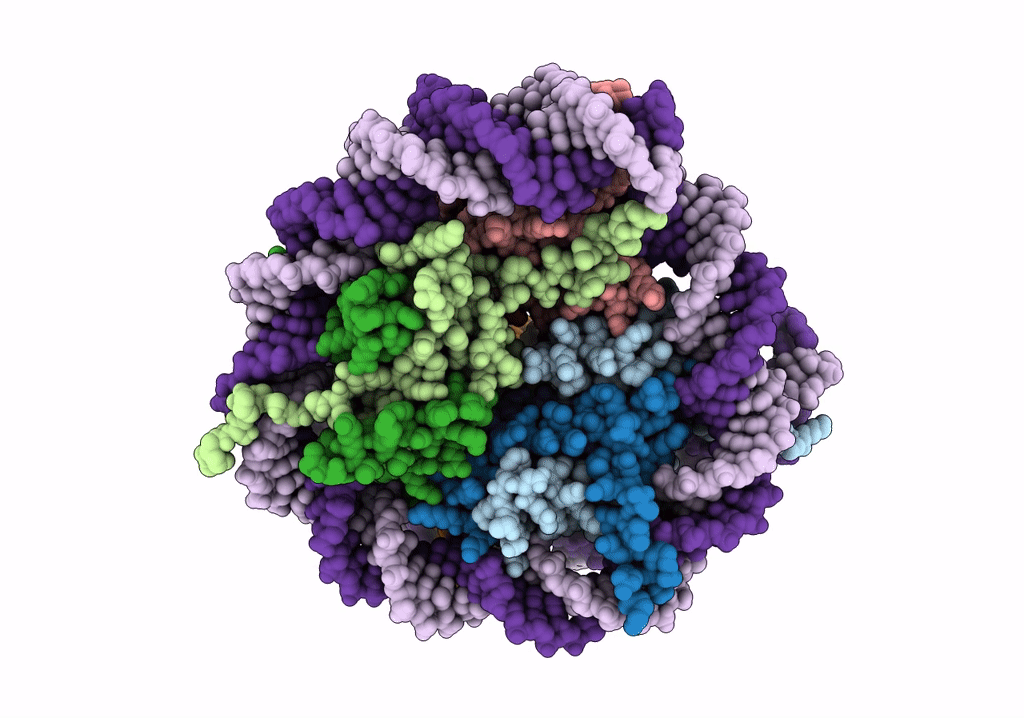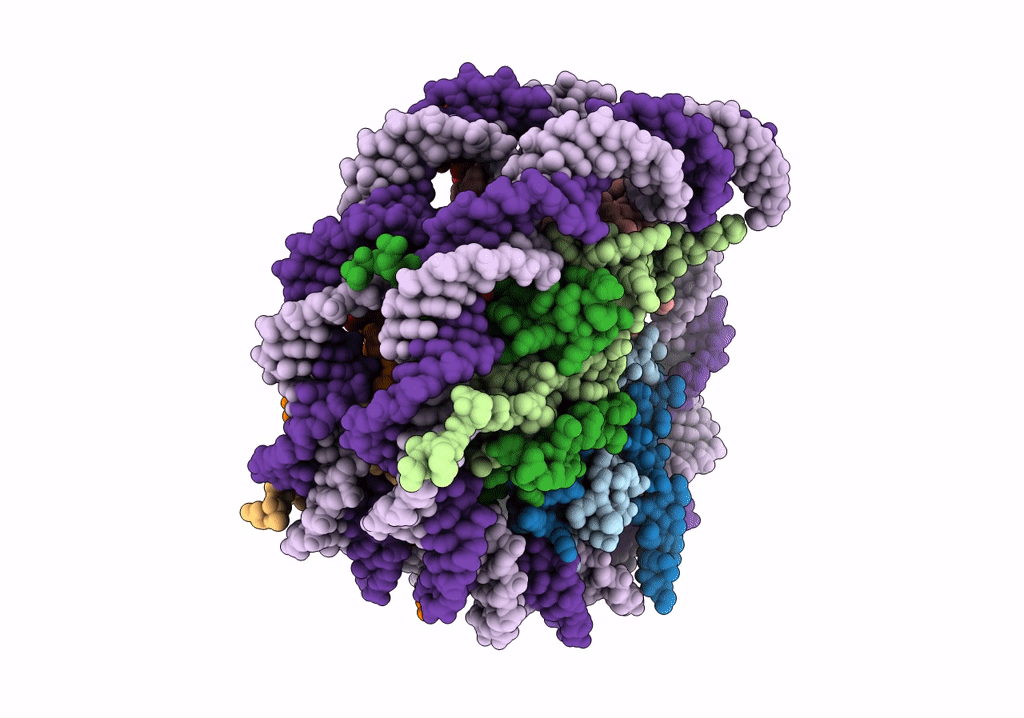
Structure of LSD2/NPAC-linker/nucleosome core particle complex: Class 1, free nuclesome
Biological Source:
Source Organism(s):
Xenopus laevis (Taxon ID: 8355)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
4.02 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE