Deposition Date
2019-02-12
Release Date
2020-03-04
Last Version Date
2024-01-24
Entry Detail
PDB ID:
6QNZ
Keywords:
Title:
Crystal structure of the site-specific DNA nickase N.BspD6I E418A Mutant
Biological Source:
Source Organism:
Bacillus sp. D6 (Taxon ID: 127889)
Host Organism:
Method Details:
Experimental Method:
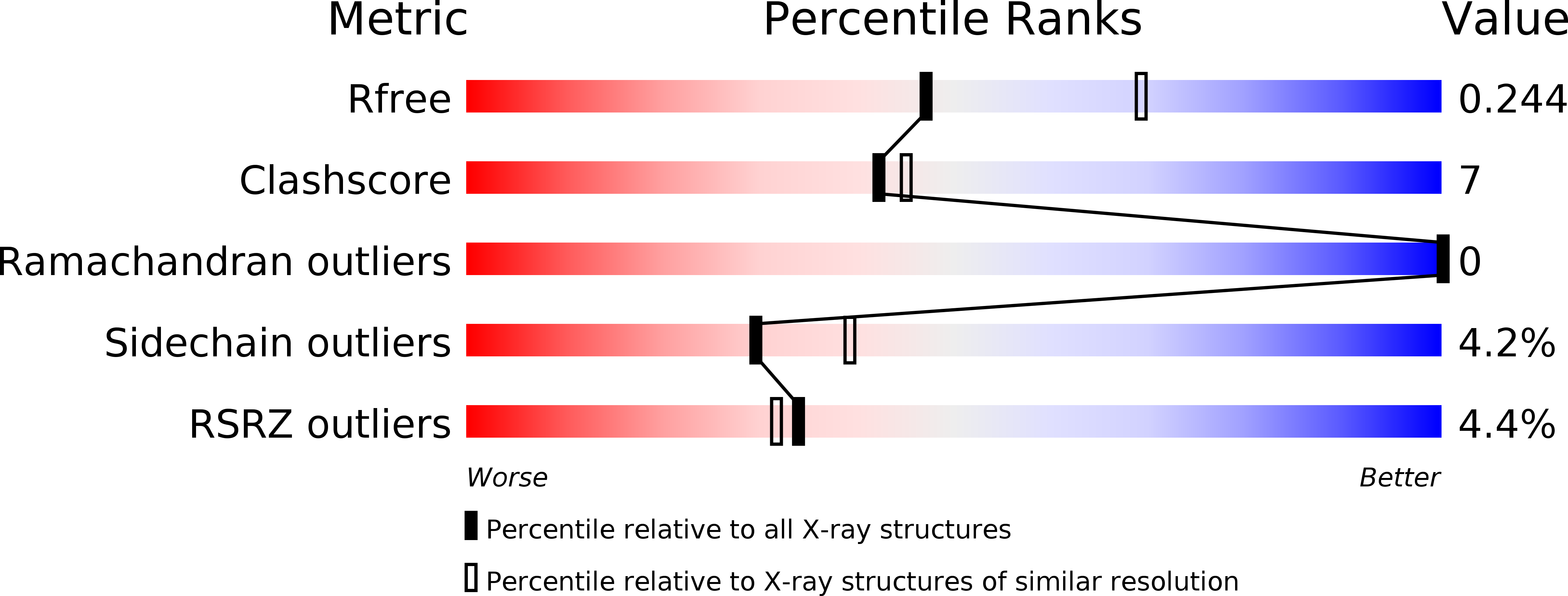
Resolution:
2.45 Å
R-Value Free:
0.24
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
P 1 21 1