Deposition Date
2019-01-30
Release Date
2019-05-15
Last Version Date
2024-10-16
Entry Detail
PDB ID:
6QKX
Keywords:
Title:
2-Naphthoyl-CoA Reductase-DiHydroNaphthoyl-CoA complex(NCR-DHNCoA co-crystallized complex)
Biological Source:
Source Organism(s):
bacterium enrichment culture clone N47 (Taxon ID: 700510)
Expression System(s):
Method Details:
Experimental Method:
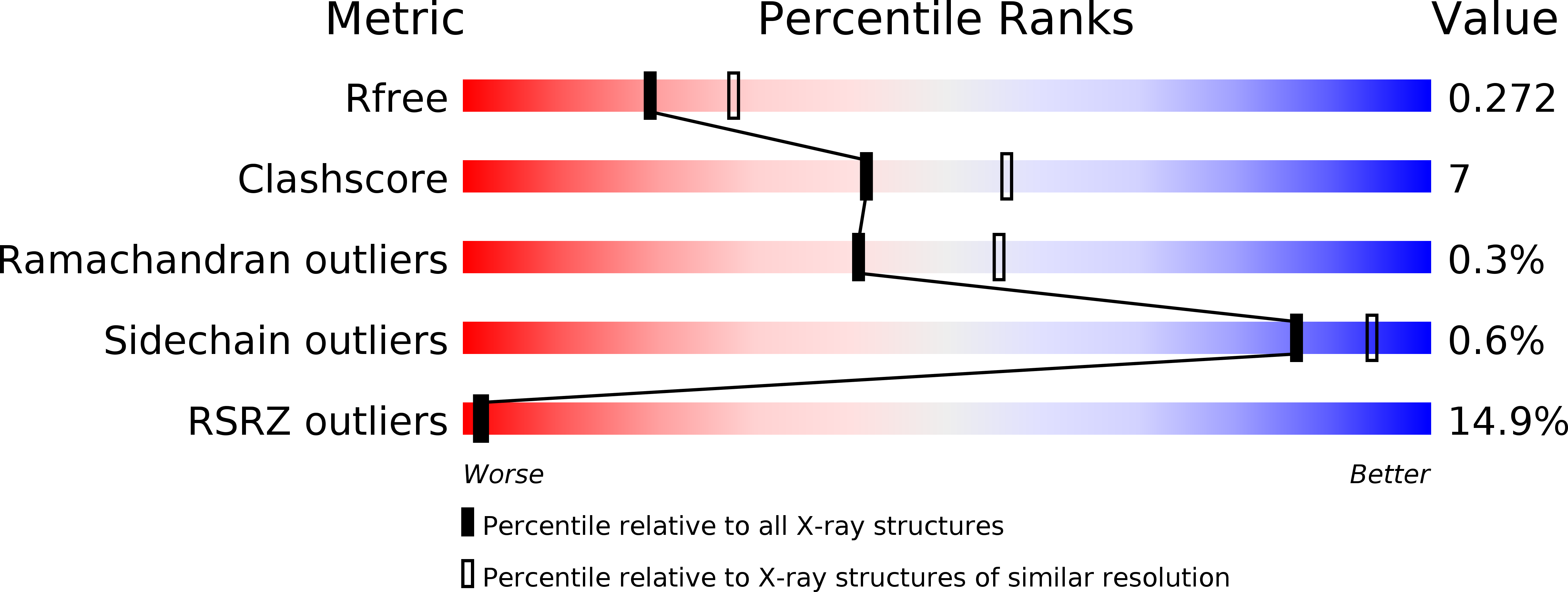
Resolution:
2.40 Å
R-Value Free:
0.27
R-Value Work:
0.22
R-Value Observed:
0.23
Space Group:
I 41